|
|
|
|
 |
RODENTS: Mice, White-Footed and Deer |
|
|
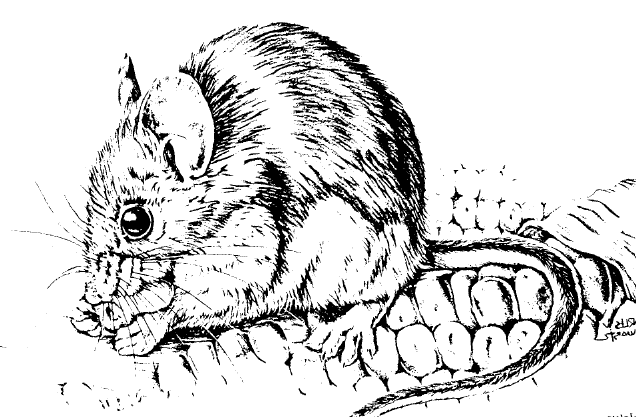
Identification
Fifteen species of native
mice of the genus Peromyscus may be found in the United
States. The two most common and widely distributed
species are the deer mouse (Peromyscus maniculatus, Fig.
1) and the white-footed mouse (P. leucopus). This
chapter will deal primarily with these species.
Collectively, all species of Peromyscus are often
referred to as “white-footed mice” or “deer mice.” Other
species include the brush mouse (P. boylei), cactus
mouse (P. eremicus), canyon mouse (P. crinitus), cotton
mouse (P. gossypinus), golden mouse (P. nuttalli), piñon
mouse (P. truei), rock mouse (P. difficilis), white-ankled
mouse (P. pectoralis), Merriam mouse (P. merriami),
California mouse (P. californicus), Sitka mouse (P.
sitkensis), oldfield mouse (P. polionotus), and the
Florida mouse (P. floridanus).
All of the Peromyscus
species have white feet, usually white undersides, and
brownish upper surfaces. Their tails are relatively
long, sometimes as long as the head and body. The deer
mouse and some other species have a distinct separation
between the brownish back and white belly. Their tails
are also sharply bicolored. It is difficult even for an
expert to tell all of the species apart.
In comparison to house
mice, white-footed and deer mice have larger eyes and
ears. They are considered by most people to be more
“attractive” than house mice, and they do not have the
characteristic mousy odor of house mice. All species of
Peromyscus cause similar problems and require similar
solutions.
Range
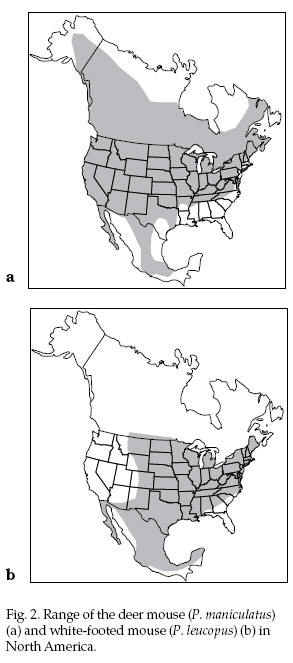 The
deer mouse is found throughout most of North America
(Fig. 2). The white-footed mouse is found throughout the
United States east of the Rocky Mountains except in
parts of the Southeast (Fig. 2). The
deer mouse is found throughout most of North America
(Fig. 2). The white-footed mouse is found throughout the
United States east of the Rocky Mountains except in
parts of the Southeast (Fig. 2).
Fig. 2. Range of the deer
mouse (P. maniculatus) (a) and white-footed mouse (P.
leucopus) (b) in North America.
The brush mouse is found
from southwestern Missouri and northwestern Arkansas
through Oklahoma, central and western Texas, New Mexico,
southwestern Colorado, Utah, Arizona, and California.
The cactus mouse is limited to western Texas, southern
New Mexico, Arizona (except the northeast portion), and
southern California. The canyon mouse occurs in western
Colorado, northwestern New Mexico, northern and western
Arizona, Utah, Nevada, southern California, southeast
Oregon, and southwestern Idaho.
The cotton mouse is found
only in the southeastern United States from east Texas
and Arkansas through southeastern Virginia. The golden
mouse occupies a similar range but it extends slightly
farther north.
The piñon mouse is found
from southwestern California through the southwestern
United States to the Texas panhandle. The rock mouse is
limited to Colorado, southeastern Utah, eastern Arizona,
New Mexico, and the far western portion of Texas. The
white-ankled mouse is found only in parts of Texas and
small areas in southern New Mexico, southern Oklahoma,
and southern Arizona.
The Merriam mouse is
limited to areas within southern Arizona. The California
mouse ranges from San Francisco Bay to northern Baja
California, including parts of the southern San Joaquin
Valley. The Sitka mouse is found only on certain islands
of Alaska and British Columbia.
The oldfield mouse is
distributed across eastern Alabama, Georgia, South
Carolina, and Florida. The Florida mouse, as its name
indicates, is found only in Florida.
Habitat
The deer mouse occupies
nearly every type of habitat within its range, from
forests to grasslands. It is the most widely distributed
and abundant mammal in North America.
The white-footed mouse is
also widely distributed but prefers wooded or brushy
areas. It is sometimes found in open areas.
The other species of
Peromyscus have somewhat more specialized habitat
preferences. For example, the cactus mouse occurs in low
deserts with sandy soil and scattered vegetation and on
rocky outcrops. The brush mouse lives in chaparral areas
of semidesert regions, often in rocky habitats.
Food Habits
White-footed and deer mice
are primarily seed eaters. Frequently they will feed on
seeds, nuts, acorns, and other similar items that are
available. They also consume fruits, insects and insect
larvae, fungi, and possibly some green vegetation. They
often store quantities of food near their nest sites,
particularly in the fall when seeds, nuts, or acorns are
abundant.
General Biology, Reproduction, and Behavior
White-footed and deer mice
are mostly nocturnal with a home range of 1/3 acre to 4
acres (0.1 to 1.6 ha) or larger. A summer population
density may reach a high of about 15 mice per acre
(37/ha).
In warm regions,
reproduction may occur more or less year-round in some
species. More typically, breeding occurs from spring
until fall with a summer lull. This is especially true
in cooler climates. Litter size varies from 1 to 8
young, but is usually 3 to 5. Females may have from 2 to
4 or more litters per year, depending on species and
climate.
During the breeding
season, female white-footed and deer mice come into heat
every fifth day until impregnated. The gestation period
is usually 21 to 23 days, but may be as long as 37 days
in nursing females. Young are weaned when they are 2 to
3 weeks old and become sexually mature at about 7 to 8
weeks of age. Those born in spring and summer may breed
that same year.
Mated pairs usually remain
together during the breeding season but may take new
mates in the spring if both survive the winter. If one
mate dies, a new one is acquired. Family groups usually
nest together through the winter. They do not hibernate
but may become torpid for a few days when winter weather
is severe.
Nests consist of stems,
twigs, leaves, roots of grasses, and other fibrous
materials. They may be lined with fur, feathers, or
shredded cloth. The deer mouse often builds its nest
underground in cavities beneath the roots of trees or
shrubs, beneath a log or board, or in a burrow made by
another rodent. Sometimes deer mice nest in aboveground
sites such as a hollow log or fencepost, or in cupboards
and furniture of unoccupied buildings.
White-footed mice spend a
great deal of time in trees. They may use aban-locating
and digging up buried seed. Formerly, much reforestation
was attempted by direct seeding of clear-cut areas, but
seed predation by deer mice and white-footed mice, and
by other rodents and birds, caused frequent failure in
the regeneration. For this reason, to reestablish
Douglas fir and other commercial timber species today,
it is often necessary to hand-plant seedlings, despite
the increased expense of this method.
Damage and Damage Identification
The principal problem
caused by white-footed and deer mice is their tendency
to enter homes, cabins, and other structures that are
not rodent-proof. Here they build nests, store food, and
can cause considerable damage to upholstered furniture,
mattresses, clothing, paper, or other materials that
they find suitable for their nest-building activities.
Nests, droppings, and other signs left by these mice are
similar to those of house mice. White-footed and deer
mice have a greater tendency to cache food supplies,
such as acorns, seeds, or nuts, than do house mice.
White-footed and deer mice are uncommon in urban or
suburban residential areas unless there is considerable
open space (fields, parks) nearby.
Both white-footed and deer
mice occasionally dig up and consume newly planted seeds
in gardens, flowerbeds, and field borders. Their
excellent sense of smell makes them highly efficient at
locating and digging up buried seed. Formerly, much
reforestation was attempted by direct seeding of
clearcut areas, but seed predation by deer mice and
white-footed mice, and by other rodents and birds,
caused frequent failure in the regeneration. For this
reason, to reestablish Douglas fir and other commercial
timber species today, it is often necessary to handplant
seedlings, despite the increased expense of this method.
In mid-1993, the deer
mouse (P. maniculatus) was first implicated as a
potential reservoir of a type of hantavirus responsible
for an adult respiratory distress syndrome, leading to
several deaths in the Four Corners area of the United
States. Subsequent isolations of the virus thought
responsible for this illness have been made from several
Western states. The source of the disease is thought to
be through human contact with urine, feces, or saliva
from infected rodents.
Legal Status
White-footed and deer mice
are considered native, nongame mammals and receive
whatever protection may be afforded such species under
state or local laws. It is usually permissible to
control them when necessary, but first check with your
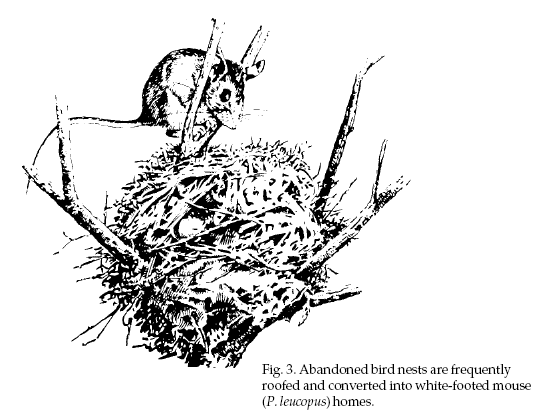
state wildlife agency. Doned bird or squirrel nests,
adding a protective “roof” of twigs and other materials
to completely enclose a bird’s nest (Fig. 3). Like deer
mice, they nest at or just below ground level or in
buildings.
Damage Prevention and Control Methods
Exclusion
Rodent-proof construction is the best and most
permanent method of preventing rodents from entering
homes, cabins, or other structures. White-footed and
deer mice require measures similar to those used for
excluding house mice. No openings larger than 1/4 inch
(0.6 cm) should be left unmodified. Mice will gnaw to
enlarge such openings so they can gain entry. For
additional information, see the chapter Rodent-proof
Construction & Exclusion Methods.
Use folded hardware cloth
(wire mesh) of 1/4 inch (0.6 cm) or smaller to protect
newly seeded garden plots. Homemade wire-screen caps or
bowls can be placed over seeded spots. Bury the edges of
the wire several inches beneath the soil. Plastic
strawberry-type baskets inverted over seeded spots serve
a similar purpose.
Habitat Modification
Store foodstuffs such as dry pet food, grass seed,
and boxed groceries left in cabins in rodent-proof
containers.
Mouse damage can be
reduced in cabins or other buildings that are used only
occasionally, by removing or limiting nesting
opportunities for mice. Remove padded cushions from
sofas and chairs and store them on edge, separate from
one another, preferably off the floor. Remove drawers in
empty cupboards or chests and reinsert them upside-down,
eliminating them as suitable nesting sites. Other such
techniques can be invented to outwit mice. Remember that
white-footed and deer mice are excellent climbers. They
frequently enter buildings by way of fireplace chimneys,
so seal off fireplaces when not in use.
When cleaning areas
previously used by mice, take precautions to reduce
exposure to dust, their excreta, and carcasses of dead
mice. Where deer mice or related species may be
reservoirs of hantaviruses, the area should be
disinfected by spraying it thoroughly with a
disinfectant or a solution of diluted household bleach
prior to beginning any sweeping, vacuuming, or handling
of surfaces or materials with which mice have had
contact. Use appropriate protective clothing, including
vinyl or latex gloves. Contact the Centers for Disease
Control (CDC) Hotline for current recommendations when
handling rodents or cleaning areas previously infested.
Frightening
There are no methods known for successfully keeping
white-footed or deer mice out of structures by means of
sound. Ultrasonic devices that are commercially sold and
advertised to control rodents and other pests have not
proven to give satisfactory control.
Repellents
Moth balls or flakes (naphthalene) may effectively
repel mice from closed areas where a sufficient
concentration of the chemical can be attained in the
air. These materials are not registered for the purpose
of repelling mice, however.
Toxicants
Anticoagulants. Anticoagulant baits such as warfarin,
diphacinone, chlorophacinone, brodifacoum, and
bromadiolone are all quite effective on white-footed and
deer mice, although they are not specifically registered
for use on these species. Brodifacoum and bromadiolone,
unlike the other anticoagulants, may be effective in a
single feeding. If baiting in and around structures is
done for house mice in accordance with label directions,
white-footed and deer mice usually will be controlled.
No violation of pesticide laws should be involved since
the “site” of bait application is the same.
Behavioral differences may
result in white-footed and deer mice carrying off and
hoarding more bait than house mice normally do. For this
reason, loose-grain bait formulations or secured
paraffin wax bait blocks may be more effective, since
these cannot be easily carried off. Cabins should be
baited before being left unoccupied. For further
information on anticoagulant baits and their use, see
the chapter House Mice.
Zinc phosphide. Various
zinc phosphide grain baits (1.0% to 2.0% active
ingredient) are registered for the control of Peromyscus
as well as voles and for post-harvest application in
orchards and at other sites. Zinc phosphide is a
single-dose toxicant, and all formulations are
Restricted Use Pesticides. Follow label directions when
applying. There are few damage situations where control
of white-footed or deer mice require the use of zinc
phosphide.
Fumigants
None are registered for white-footed or deer mice.
Because of the species’ habitat, there are few
situations where fumigation would be practical or
necessary.
Trapping
Ordinary mouse snap traps, sold in most grocery and
hardware stores, are effective in catching white-footed
and deer mice. Bait traps with peanut butter, sunflower
seed, or moistened rolled oats. For best results, use
several traps even if only a single mouse is believed to
be present. Set traps as you would for house mice:
against walls, along likely travel routes, and behind
objects. Automatic traps designed to live-capture
several house mice in a single setting also are
effective against white-footed and deer mice. They
should be checked frequently to dispose of captured mice
in an appropriate manner: euthanize them with carbon
dioxide gas in a closed container, or release them alive
into an appropriate location where they won’t cause
future problems. For further details on trapping, see
House Mice.
Other Methods
Recent research has revealed the possibility that
supplemental feeding at time of seeding can increase
survival of conifer seed by reducing predation by deer
mice, although the tests were not carried out to
germination.
Sunflower seed, and a
combination of sunflower and oats, were applied along
with Douglas fir and lodgepole pine seed in ratios
ranging from two to seven alternate foods to one conifer
seed. Significantly more conifer seeds survived mouse
predation for the 6and 9-week test periods than without
the supplemental feeding. For further details on the
experimental use of this technique, see Sullivan and
Sullivan (1982a and 1982b).
Economics of Damage and Control
Damage by both
white-footed and deer mice is usually a nuisance. When
mice destroy furniture or stored materials, the cost of
such damage depends upon the particular circumstances.
The greatest economic impact of deer mice is their
destruction of conifer seed in forest reseeding
operations. In west coast forest areas, Peromyscus seed
predation has resulted in millions of dollars worth of
damage and has been documented to have been a serious
problem since the early 1900s. New efficacious,
cost-effective methods of reducing this seed predation
are needed.
Acknowledgments
Much of the information in
this chapter was taken from Marsh and Howard (1990) and
from Schwartz and Schwartz (1981).
Figures 1 through 3 from
Schwartz and Schwartz (1981).
For
Additional Information
Burt, W. H., and R. P.
Grossenheider. 1976. A field guide to the mammals, 3d
ed. Houghton Mifflin Co., Boston. 289 pp.
Clark, J. P. 1986.
Vertebrate pest control handbook. California Dep. Food
Agric. Sacramento. 610 pp.
Everett, R. L., and R.
Stevens. 1981. Deer mouse consumption of bitterbrush
seed treated with four repellents. J. Range Manage.
34:393-396.
Howard, W. E., R. E.
Marsh, and R. E. Cole. 1968. Food detection by deer mice
using olfactory rather than visual cues. An. Behav.
16:13-17.
Howard, W. E., R. E.
Marsh, and R. E. Cole. 1970. A diphacinone bait for deer
mouse control. J. For. 68:220-222.
King, J. A., ed. 1968.
Biology of Peromyscus (Rodentia). Am. Soc. Mammal.,
Spec. Publ. 2. 539 pp.
Kirkland, G. L., Jr., and
J. N. Layne, eds. 1989. Advances in the study of
Peromyscus (Rodentia). Texas Tech. Univ. Press, Lubbock.
366 pp.
Marsh, R. E., and W. E.
Howard. 1990. Vertebrate pests. Pages 771-831 in A.
Mallis, ed. Handbook of pest control, 7th ed. Franzak
and Foster Co., Cleveland, Ohio.
Schwartz, C. W., and E. R.
Schwartz. 1981. The wild mammals of Missouri, rev. ed.
Univ. Missouri Press, Columbia. 356 pp.
Sullivan, T. P., and D. S.
Sullivan. 1982a. The use of alternative foods to reduce
lodgepole pine seed predation by small mammals. J. Appl.
Ecol. 19:33-45.
Sullivan, T. P., and D. S.
Sullivan. 1982b. Reducing conifer seed predation by use
of alternative foods. J. For. 80:499-500.
Taitt, M. J. 1981. The
effect of extra food on small rodent populations: I.
Deer mice (Peromyscus maniculatus). J. An. Ecol.
50:111-124.
Editors
Scott E. Hygnstrom; Robert
M. Timm; Gary E. Larson
PREVENTION AND CONTROL OF
WILDLIFE DAMAGE — 1994
Cooperative Extension
Division Institute of Agriculture and Natural Resources
University of Nebraska -Lincoln
United States Department
of Agriculture Animal and Plant Health Inspection
Service Animal Damage Control
Special
thanks to:
Clemson University
|