|
|
|
|
 |
RODENTS: House Mice |
|
|
Damage
Prevention and Control Methods
- Exclusion
- Seal all openings
larger than 1/4 inch (0.6 cm) wide.
- Habitat
Modification
- Good sanitation
practices reduce sources of food, water, and
shelter.
- Store foodstuffs
in rodent-proof structures or containers.
- Control weeds and
remove debris from around structures.
- Frightening
- Ultrasonic devices
have not been proven to control mice.
- Repellents
- Ro-pel®
- Moth flakes
(naphthalene) not specifically registered, but may
be of some value.
- Toxicants
- Anticoagulant
rodenticides (slowacting chronic-type toxicants).
Brodifacoum (Talon®). Bromadiolone (Maki®, Contrac®).
Chlorophacinone (RoZol®). Diphacinone (Ditrac®).
Pindone(Pival®, Pivalyn®). Warfarin (Final® and
others).
- Toxicants other
than anticoagulants (may be acute or chronic
poisons). Bromethalin (Assault®, Vengeance®).
Cholecalciferol (Quintox®). Zinc phosphide (Ridall
Zinc®, ZP®).
- Fumigants
- Practical use is
limited to structures, containers, and commodities;
for use only by trained personnel.
- Trapping
- Snap traps.
- Live traps
(Sherman-type, Ketch-All®, Tin Cat®, and others).
- Glue boards.
- Other Methods
- Predators: dogs
and cats are of limited value in some situations.
Identification
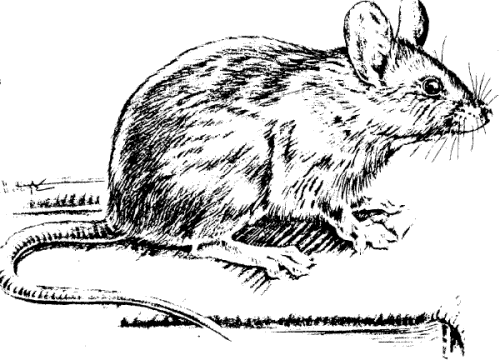
The house mouse (Mus
musculus, Fig. 1) is a small, slender rodent that has a
slightly pointed nose; small, black, somewhat protruding
eyes; large, sparsely haired ears; and a nearly hairless
tail with obvious scale rings. House mice are considered
among the most troublesome and economically important
rodents in the United States.
Adult house mice weigh
about 2/5 to 4/5 ounce (11 to 22 grams). They are
generally grayish brown with a gray or buff belly.
Similar mice include the white-footed mice and jumping
mice (which have a white belly), and harvest mice (which
have grooved upper incisor teeth). For more details on
species identification, see a field guide such as that
by Burt and Grossenheider (1976).
Native to central Asia,
this species arrived in North America with settlers from
Europe and from other points of origin. A very adaptable
species, the house mouse often lives in close
association with humans and therefore is termed one of
the “commensal” rodents along with Norway and roof rats.
House mice are much more common in residences and
commercial structures than are rats. Brooks (1973)
regards them to be the most common mammal in cities,
next to humans.
Range
Following their arrival on
colonists’ ships, house mice spread across North America
and are now found in every state, including coastal
areas of Alaska, and in the southern parts of Canada.
Habitat
House mice live in and
around homes, farms, commercial establishments, and in
open fields and agricultural lands. At times they may be
found living far from human settlements, particularly
where climates are moderate. The onset of cold weather
each fall in temperate regions may cause mice to move
into structures in search of shelter and food.
Food
Habits
House mice eat many types
of food but prefer seeds and grain. They are not
hesitant to eat new foods and are considered “nibblers,”
sampling many kinds of items that may exist in their
environment. Foods high in fat, protein, or sugar may be
preferred even when grain and seed are present. Such
items include bacon, chocolate candies, butter, and
nutmeats.
Unlike Norway and roof
rats, house mice can survive with little or no free
water, although they readily drink water when it is
available. They obtain their water from the food they
eat. An absence of liquid water or food of adequate
moisture content in their environment may reduce their
breeding potential
General
Biology, Reproduction, and Behavior
House mice are mainly
nocturnal, although at some locations considerable
daytime activity may be seen. Seeing mice during
daylight hours does not necessarily mean that a high
population is present, although this is usually true for
rats.
Mice have poor eyesight,
relying on their hearing and their excellent senses of
smell, taste, and touch. They are considered
color-blind; therefore, for safety reasons, baits can be
dyed distinctive colors without causing avoidance by
mice, as long as the dye does not have an objectionable
taste or odor.
House mice may burrow into
the ground in fields or around structures when other
shelter is not readily available. Nesting may occur in
the ground or in any sheltered location. Nests are
constructed of shredded fibrous materials such as paper,
burlap, or other similar items, and generally have the
appearance of a “ball” of material loosely woven
together. They are usually 4 to 6 inches (10.2 to 15.2
cm) in diameter.
Litters of 5 or 6 young
are born 19 to 21 days after mating, although females
that conceive while still nursing may have a slightly
longer gestation period. Mice are born hairless and with
their eyes closed. They grow rapidly, and after 2 weeks
they are covered with hair and their eyes and ears are
open. They begin to make short excursions from the nest
and eat solid food at 3 weeks. Weaning soon follows, and
mice are sexually mature at 6 to 10 weeks of age.
Mice may breed year-round,
but when living outdoors, they breed mostly in spring
and fall. A female may have 5 to 10 litters per year.
Mouse populations can therefore grow rapidly under good
conditions, although breeding and survival of young
decline markedly when population densities become high.
House mice have physical
capabilities that enable them to gain entry to
structures by gnawing, climbing, jumping, and swimming.
For more detailed information on their physical
abilities and the resulting need to design rodent-proof
structures, see the chapter Rodent-Proof Construction
and Exclusion Methods.
Studies indicate that
during its daily activities, a mouse normally travels an
area averaging 10 to 30 feet (3 m to 9 m) in diameter.
Mice seldom travel farther than this to obtain food or
water. Because of their limited movement and feeding
behavior, both of which differ from those of commensal
rats, they are much more difficult to control in some
situations.
Mice constantly explore
and learn about their environment, memorizing the
locations of pathways, obstacles, food and water,
shelter, and other elements in their domain. They
quickly detect new objects in their environment but,
unlike rats, do not fear them. Thus, they will almost
immediately enter bait stations and sample new foods
(baits). The degree to which mice consume a particular
food depends on the flavor of the food in addition to
its physiological effect. Mice may reject baits simply
because they do not taste as good as other available
foods.
If the bait contains
poison or some other substance that produces an ill
effect (but not death) within a few hours, the bait will
often become associated with the illness. Bait shyness
can persist for weeks or months and may be transferred
to nontoxic foods of similar types. Prebaiting, that is,
training mice to feed repeatedly on nontoxic bait for a
period of days prior to applying the toxicant in the
bait, will largely prevent sublethal doses and thus bait
shyness. It will also reduce the number of mice left to
be bait shy. Prebaiting is especially recommended with
zinc phosphide baits. All of the other toxic baits
currently registered for house mice are chronic or
slow-acting. Because of this slow action, the mice’s
subsequent illness is not associated with the bait even
if a sublethal dose is consumed; thus, bait shyness does
not usually occur. These baits, in effect, serve as
their own prebait.
Damage and
Damage Identification
When house mice live in or
around structures, they almost always cause some degree
of economic damage. In homes and commercial buildings,
they may feed on various stored food items or pet foods.
In addition, they usually contaminate foodstuffs with
their urine, droppings, and hair. On farms, they may
cause damage to feed storage structures and feed
transporting equipment. A single mouse eats only about 3
grams of food per day (8 pounds [3.6 kg] per year) but
destroys considerably more food than it consumes because
of its habit of nibbling on many foods and discarding
partially eaten items.
House mice living in
fields may dig up and feed on newly planted grain, or
may cause some damage to crops before harvest. But
losses in stored foods are considerably greater. Mice
commonly damage containers and packaging materials in
warehouses where food and feeds are stored. Much of this
loss is due to contamination with droppings and urine,
making food unfit for human consumption.
House mice cause
structural damage to buildings by their gnawing and
nest-building activities. In livestock confinement
facilities and similar structures, they may quickly
cause extensive damage to insulation inside walls and
attics. Such damage also occurs in homes, apartments,
offices, and commercial buildings but usually at a
slower rate because mouse populations in such structures
are smaller. House mice often make homes in large
electrical appliances, and here they may chew up wiring
as well as insulation, resulting in short circuits which
create fire hazards or other malfunctions that are
expensive to repair. Mice may also damage stored items
in attics, basements, garages, or museums. Damaged
family heirlooms, paintings, books, documents, and other
such items may be impossible to replace.
Among the diseases mice or
their parasites may transmit to humans are salmonellosis
(food poisoning), rickettsialpox, and lymphocytic
choriomeningitis. Mice may also carry leptospirosis,
ratbite fever, tapeworms, and organisms that can cause
ringworm (a ungal skin disease) in humans. They have
also been found to act as reservoirs or transmitters of
diseases of veterinary importance, such as swine
dysentery, a serious bacterial disease of swine often
called “bloody scours.”
Mouse Sign
The presence of house mice
can be determined by a number of signs described below:
Droppings may be found
along runways, in feeding areas, and near shelter.
Differentiating between mouse droppings and those of
certain insects may be difficult. Mouse droppings are
about 1/4 inch (0.6 cm) long, whereas those of
cockroaches are usually 1/8 to 1/4 inch (0.3 to 0.6 cm)
long and under a magnifying glass show distinct
longitudinal ridges and squared-off ends. In comparison,
droppings of bats contain insect fragments and are more
easily crushed between the fingers.
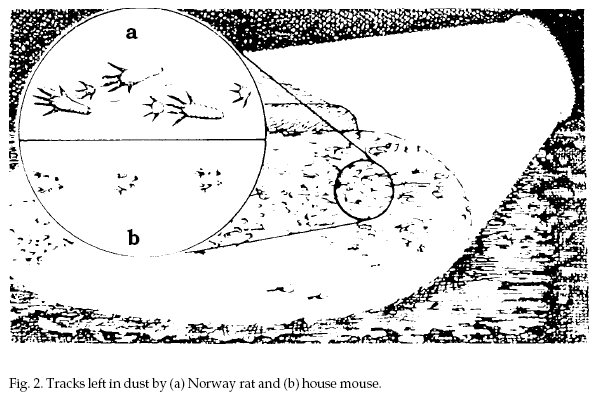
Tracks, including
footprints or tail marks, may be seen on dusty surfaces
or in mud (Fig. 2). A tracking patch made of flour,
rolled smooth with a cylindrical object, can be placed
in pathways overnight to determine if rodents are
present.
Urine, both wet and dry,
will fluoresce under ultraviolet light, although so will
some other materials. Urine stains may occur along
travelways or in feeding areas.
Smudge marks
(rub marks) may occur on beams, rafters, pipes, walls,
and other parts of structures. They are the result of
oil and dirt rubbing off mice’s fur along frequently
traveled routes (Fig. 3). They may be less apparent than
rub marks left by rats.
Gnawing may
be visible on doors, ledges, in corners, in wall
material, on stored materials, or on other surfaces
wherever mice are present. Fresh accumulations of wood
shavings, insulation, and other gnawed material indicate
active infestations. Size of entry holes (often 1 1/2
inches [3.8 cm] in diameter or less for mice, 2 inches
[5 cm] or larger for rat) or tooth marks can be used to
distinguish rat gnawing from mouse gnawing. Mice keep
their paired incisor teeth, which grow continuously,
worn down by gnawing on hard surfaces and by working
them against each other
Sounds such
as gnawing, climbing in walls, running across the upper
surface of ceilings, and squeaks are common where mice
are present.
Visual sightings
of mice may be possible during daylight hours, and mice
also can be seen after dark with the aid of a flashlight
or spotlight.
Nests
frequently are found when cleaning garages, closets,
attics, basements, and outbuildings where mice are
present. They consist of fine, shredded fibrous
materials
Odors may
indicate the presence of house mice. A characteristic
musky odor is a positive indication that house mice are
present, and this odor can be used to differentiate
their presence from that of rats.
Estimating Mouse Numbers
Mouse sign and visual
sightings are of limited value in accurately estimating
mouse numbers, but they are the simplest and often the
only practical method available. Search premises
thoroughly when looking for mice. In structures,
searches should include attics, basements, around
foundations, crawl spaces, and behind and under stored
materials.
One method to detect the
presence of mice is to make nontoxic tracking-dust
patches of flour or talc at 20- to 30-foot (6- to 9-m)
intervals throughout a structure. The number of patches
showing tracks after 24 hours, and the abundance of
tracks in each patch, indicate the size of the
population. Because house mice, unlike rats, do not
travel far from their nests or shelter, the percentage
of patches showing tracks is a good indicator of the
relative size and distribution of the mouse population.
Snap trapping is also an
excellent way to determine the presence of mice. A
relative index of mouse abundance can be calculated from
the number of mice trapped for a certain number of traps
set during 1 or more nights (for example, 35 mice caught
per 100 trap nights).
Legal Status
House mice are not
protected by law. They may be controlled using any
pesticide registered by federal or state authorities for
this purpose, or they may be controlled by use of
mechanical methods such as traps.
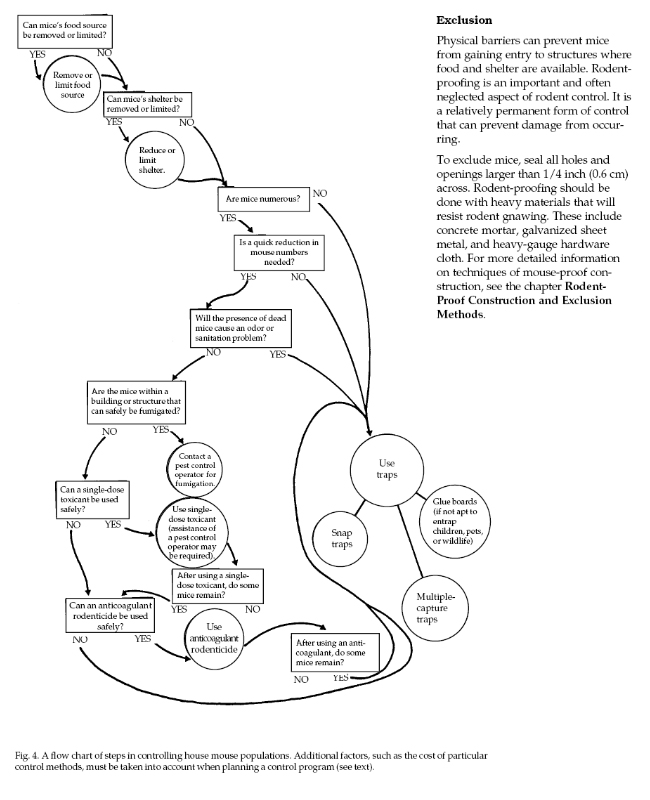
Damage Prevention and Control Methods
Effective prevention and
control of house mouse damage involves three aspects:
rodent-proof construction, sanitation, and population
reduction by means of traps, toxicants, or fumigants.
The first two are useful as preventive measures, but
when a house mouse infestation already exists, some form
of population reduction is almost always necessary. A
flow chart outlining steps in controlling house mice is
found in figure 4.
Control of house mice
differs in important ways from the control of Norway or
roof rats. Mice are smaller and therefore can enter
narrower openings, making rodent-proofing more
difficult. They have limited areas of movement (home
range) and require little or no free water. While having
a reproductive capability that is higher than that of
rats, house mice are usually less sensitive (often far
less sensitive) to many rodenticides. Persons who do not
take these differences into account when attempting
house mouse control may expect poor results.
After rats are controlled
at a given location, house mice may increase in numbers
by moving in from elsewhere or by reproduction. This may
be expected because habitats suitable for rats are
usually even more suitable for mice. One should
anticipate that following rat control, the potential for
house mouse problems may increase, and control measures
should be taken before mouse numbers reach high levels.
Habitat Modification
Sanitation, which includes
good housekeeping practices and proper storage and
handling of food materials, feed, and garbage, is often
stressed as a method of rodent control. Unfortunately,
even the best sanitation will not eliminate house mice.
It will, however, aid in control by permitting easier
detection of mouse sign, increasing effectiveness of
traps and baits by reducing competing food items, and by
preventing mice from flourishing and reaching high
populations.
Although house mice are
less dependent upon humans for their existence than are
Norway rats, they are much more adaptable to living with
people. They require very little space and only small
amounts of food. Mice have been known to inhabit
buildings even before construction has been complete,
living off the crumbs and scraps of worker’s lunches. In
offices, mice may live behind cabinets or furniture and
feed on scraps or crumbs from lunches and snacks and on
cookies or candy bars kept in desks. In homes, they may
find ample food in kitchens, and in the garage they will
eat sacked or spilled pet food, grass seed, or insects
such as cockroaches. Thus, no matter how good the
sanitation, most buildings in which food is stored,
prepared, or consumed will support at least a few mice.
For this reason, a constant watch must be kept for mice
that may invade the premises.
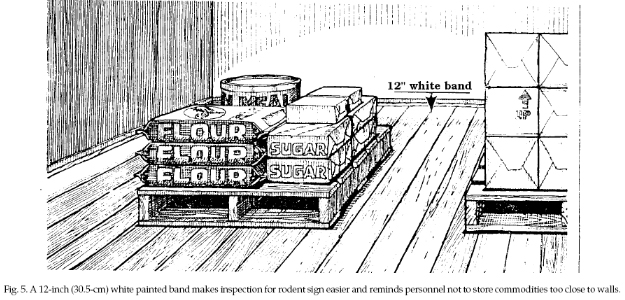
Where possible, store bulk
foods in rodent-proof containers or rooms. Stack sacked
or boxed foods in orderly rows on pallets in a way that
allows for thorough inspection for evidence of mice. In
such storage areas, keep stored materials away from
walls. A 12-inch (30.5-cm) white band painted on the
floor next to the wall serves as a reminder to keep
items away from walls. It also will allow you to detect
rodent droppings or other sign more easily (Fig. 5).
Sweep floors frequently to permit ready detection of
fresh droppings.
When storing foods or feed
on pallets, keep in mind that mice can jump up more than
12 inches (30.5 cm) from a flat surface. They are also
good climbers and can walk up surfaces such as wood or
concrete (unless the surfaces have a slick finish). Mice
can live for considerable periods of time within a
pallet of feed without coming down to the floor.
Regular removal of debris
and control of weeds from around structures will reduce
the amount of shelter available to rodents. In some
instances, a strip of heavy gravel placed adjacent to
building foundations or other structures will reduce
rodent burrowing at these locations. In any event, keep
the perimeter of buildings and other structures clean of
weeds and debris (including stacked lumber, firewood,
and other stored materials) to discourage rodent
activity and to allow easier detection of rodent sign.
Frightening
Mice are somewhat wary
animals and can be frightened by unfamiliar sounds or
sounds coming from new locations. Most rodents, however,
can quickly become accustomed to new sounds heard
repeatedly.
For years, devices that
produce ultrasonic sound that is claimed to control
rodents have come and gone on the market. There is
little evidence to suggest that rodents’ responses to
nonspecific, high-frequency sound is any different from
their response to sound within the range of human
hearing.
What is known about
rodents and sound?
—Unusually loud, novel, or
ultrasonic sounds, which rodents can hear, will frighten
them and may cause temporary avoidance lasting from a
few minutes to a few weeks.
What is known about
ultrasonic sound?
—It is very directional
and does not travel around corners well; thus, sound
shadows or voids are created.
—Ultrasound does not
travel very far. It loses its intensity rapidly as it
leaves the source.
—Ultrasound has not been
shown to drive established rodents out of buildings or
areas, nor has it been proven to cause above-normal
mortality in their populations. While it is possible to
cause convulsions or permanent physiological damage to
rodents with ultrasound, the intensity of such sounds
must be so great that damage to humans or domestic
animals would also be likely. Commercial ultrasonic pest
control devices do not produce sound of such intensity.
Recent tests of commercial
ultrasonic devices have indicated that rodents may be
repelled from the immediate area of the ultrasound for a
few days, but then will return and resume normal
activities. Other tests have shown the degree of
repellency to depend upon the particular ultrasonic
frequencies used, their intensity, and the preexisting
condition of the rodent infestation. Ultrasonic sound
has very limited usefulness in rodent control. The
advertising claims for many commercial devices are
unsubstantiated by scientific research. Since commercial
ultrasonic devices are often expensive and of
questionable effectiveness, they cannot be recommended
as a solution to rodent problems.
Repellents
Rodents find some types of
tastes and odors objectionable, but chemical repellents
are seldom a practical solution to mouse infestations.
Substances such as moth balls (naphthalene) or household
ammonia, in sufficient concentration, may have at least
temporary effects in keeping mice out of certain
enclosed areas. These are not specifically registered by
the EPA as mouse repellents, however.
Ro-pel® is registered for
use in repelling house mice and other rodents from
gnawing on trees, poles, fences, shrubs, garbage, and
other objects. Little information is currently available
on its effectiveness against house mice.
Other solutions to rodent
problems, including rodent-proof construction and
methods of population reduction, are usually more
permanent and cost-effective than the use of repellents.
Toxicants
Rodenticides were formerly
classified into two groups, single-dose (acute)
toxicants and multiple-dose (chronic) toxicants.
However, the complexity in mode of action of newer
rodenticides makes these classifications outdated. A
classification into two groups, the first group
including all anticoagulants and the second group all
other compounds (“non-anticoagulants”), is currently
more useful.
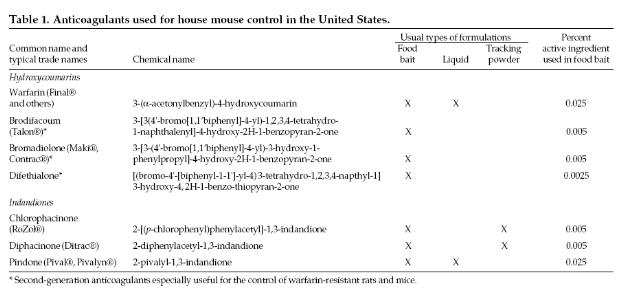
Anticoagulants
(slow-acting, chronic toxicants). House mice are
susceptible to all of the various anticoagulant
rodenticides (Table 1), but they are generally less
sensitive (often far less sensitive) to the active
ingredients than are Norway or roof rats. It usually
requires a few more feedings to produce death with the
first-genera-tion anticoagulants (such as warfarin,
diphacinone, and chlorophacinone) than with the
second-generation anticoagulants (such as brodifacoum
and bromadiolone). All anticoagulants provide good to
excellent house mouse control when prepared in
acceptable baits. A new second-generation anticoagulant,
difethialone, is presently being developed and EPA
registration is anticipated in the near future. The
characteristics of the various anticoagulant
rodenticides are described further under Anticoagulants
in the Pesticides section, and in the chapter Norway
Rats.
Because of their
similarity in mode of action, all anticoagulant baits
are used in a similar fashion. Label directions commonly
instruct the user to “maintain a continuous supply of
bait for 15 days or until feeding ceases,” thus ensuring
that the entire mouse population has ample opportunity
to ingest a lethal dose of the bait. Anticoagulants have
the same effect on nearly all warm-blooded animals, but
the sensitivity to these toxicants varies among species.
If misused, anticoagulant rodenticides can be lethal to
nontarget animals such as dogs, pigs, and cats.
Additionally, residues of anticoagulants which are
present in the bodies of dead or dying rodents can cause
toxic effects to scavengers and predators. In general,
however, the secondary poisoning hazard from
anticoagulants is relatively low.
Brodifacoum and
bromadiolone baits, because of their potential to be
lethal in a single feeding, can be more effective than
the other anticoagulants in certain situations.
Chlorophacinone (RoZol®) and diphacinone (Ditrac®) are
similar to each other in potency and are more toxic than
the anticoagulant compounds developed earlier. Thus,
they are formulated at lower concentrations.
Chlorophacinone and diphacinone may kill some mice in a
single feeding, but multiple feedings are needed to give
adequate control of a mouse population.
Pindone (Pival®, Pivalyn®)
is also less potent than chlorophacinone or diphacinone,
and is similar to warfarin in effectiveness against
house mice. It has some properties that resist insects
and growth of mold in prepared baits.
Warfarin (Final® and other
trade names) was the first marketed anticoagulant and
is, therefore, the best known and most widely used. It
is effective against house mice, although some warfarin
contains small quantities of contaminants that
apparently can reduce bait acceptance. This has been
resolved with the development of encapsulated warfarin.
Anticoagulant Resistance.
Within any population of house mice, some individuals
are less sensitive to anticoagulants than others. Where
anticoagulants have been used over long periods of time
at a particular location, there is an increased
potential for the existence of a population that is
somewhat resistant to the lethal effects of the baits.
Such resistant populations of house mice have been
identified at a number of locations throughout the
United States. Although not common, resistance may be
underestimated because relatively few resistance studies
have been conducted on house mice. Nevertheless,
resistance is of little consequence in the control of
house mice with the newer rodenticides available. When
anticoagulant resistance to the first-generation
anticoagulants is known or suspected, use of these
compounds should be avoided in favor of the
second-generation anticoagulants or one of the
non-anticoagulant products.
Anticoagulant Bait
Failure.
Resistance is only one
(and perhaps the least likely) reason for failure in the
control of mice with anticoagulant baits. Control with
baits that are highly accepted may fail for one or more
of the following reasons:
—Too short a period of
bait exposure. —Insufficient bait and insufficient
replenishment of bait (none remains from one baiting to
the next). —Too few bait stations and/or too far apart.
For mice, stations should be within 6 feet (2 m) of one
another in areas where mice are active. —Too small a
control area, permitting mice to move in from untreated
adjacent areas. —Genetic resistance to the
anticoagulant. Although this is unlikely, it should be
suspected if about the same amount of bait is taken
daily for several weeks.
--Reasons for failure to
achieve control with anticoagulant baits that are poorly
accepted: —Poor bait choice, or bait formulated
improperly. Other foods are more attractive to the mice.
—Improperly placed bait stations. Other foods are more
convenient to the mice. —Abundance of other food
choices.
—Tainted bait: the bait
has become moldy, rancid, insect-infested, or
contaminated with other material that reduces
acceptance. Discard old bait periodically, and replace
it with fresh. Occasionally, mice accept bait well and
an initial population reduction is successful. Then bait
acceptance appears to stop although some mice remain. In
such instances, it is likely that the remaining mice
never accepted the bait, either because of its
formulation or placement. The best strategy is to switch
to a different bait formulation, place baits at
different locations, and/ or use other control methods
such as traps.
Other Rodenticides. The
older rodenticides, formerly referred to as acute
toxicants, such as arsenic trioxide, phosphorus,
strychnine, and Compound 1080, are no longer registered
for house mice. Newer rodenticides are much more
effective and have resulted in the phasing out of these
older materials over the last 20 years.
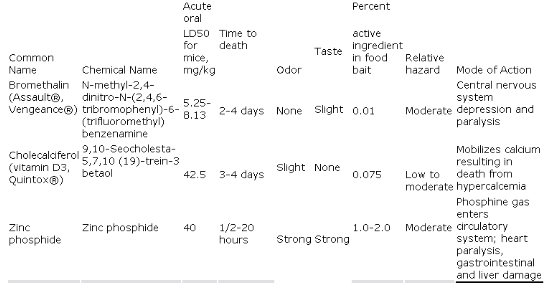
At present, three
non-anticoagulant rodenticides (Table 2) are registered
by EPA against house mice: bromethalin, cholecalciferol
(vitamin D3), and zinc phosphide. All are potentially
useful for controlling anticoagulant-resistant
populations of house mice.
Of these active
ingredients, bromethalin and cholecalciferol are
formulated to serve as chronic rodenticides, applied so
that house mice will have the opportunity to feed on the
baits one or more times over the period of one to
several days. Bait acceptance is generally good when
formulations appropriate for house mice are selected.
Zinc phosphide differs from the other two compounds in
that prebaiting (offering mice similar but nontoxic bait
prior to applying the zinc phos-phide-treated bait) is
recommended to increase bait acceptance. Zinc phosphide
baits are not designed to be left
Table 2. Other
(non-anticoagulant) rodenticides used to control house
mice in the United States.
available to mice for more
than a few days, as continued exposure is likely to
result in bait shyness within the population. Be sure to
follow label recommendations on any specific product to
achieve best success.
Bait Selection and
Formulation
Oatmeal, ground or rolled
wheat, rolled barley, ground or rolled milo, and corn
have been successfully used as chief ingredients of
toxic baits for house mice. Grass seed, such as whole
canary grass seed (Phalaris canarienses), is often
highly accepted by house mice and can be very effective
as a principal bait ingredient. In general, the fresher
the bait, the better it will be accepted by mice. Rodent
baits should always be made from high-quality food
materials, and baits should be replaced or replenished
regularly.
Food preferences may vary
among mouse populations and individuals. Bait materials
similar to foods mice are accustomed to eating are often
a good choice, particularly if their normal foods are
limited or can be made less available to them. In past
years, many people involved in house mouse control
preferred to mix their own baits so as to tailor them to
the food preference of a specific mouse population.
Today, there is a wide selection of ready-to-use baits
which are commercially available. It is still important,
particularly in moderate-to large-scale mouse control
programs, to check for differences in bait acceptance
among candidate baits prior to investing time and money
in a specific bait product. Place about 1/2 ounce (14 g)
of each of several ready-to-use baits about 4 inches (10
cm) apart in several locations where mice are present.
Check baits the next day to see which ones are
preferred.
 Ready-to-use
baits come in a variety of formulations. Grain-based
baits in a loose meal or pelleted form are available in
bulk or packaged in small plastic, cellophane, or paper
“place packs” (Fig. 6). These packets keep bait fresh
and make it easy to place baits into burrows, walls, or
other locations. Mice will gnaw into these bags to feed
on an acceptable bait. Pelleted baits can more easily be
carried by mice to other locations. Such hoarding of
food by mice is not uncommon. It may result in amounts
of bait being moved to places where it is undetected or
difficult to recover and may, if accessible, be
hazardous to nontarget species. On the other hand,
pelleted bait avoids some problems common to loose baits Ready-to-use
baits come in a variety of formulations. Grain-based
baits in a loose meal or pelleted form are available in
bulk or packaged in small plastic, cellophane, or paper
“place packs” (Fig. 6). These packets keep bait fresh
and make it easy to place baits into burrows, walls, or
other locations. Mice will gnaw into these bags to feed
on an acceptable bait. Pelleted baits can more easily be
carried by mice to other locations. Such hoarding of
food by mice is not uncommon. It may result in amounts
of bait being moved to places where it is undetected or
difficult to recover and may, if accessible, be
hazardous to nontarget species. On the other hand,
pelleted bait avoids some problems common to loose baits
— settling out of
different-sized particles during shipment and uneven
mixing of the toxicant. Pellets are easily manipulated
by mice, increasing the attractiveness of this form of
bait.
Anticoagulant baits have
also been formulated into wax and extruded blocks (Fig.
7). These are particularly useful where moisture may
cause loose grain baits to spoil. Mice accept paraffin
block baits less readily than loose or pelleted grain
baits, but acceptance of extruded bait blocks is high.
Where no water is
available, water or food items of high moisture content
are often more readily accepted than dry baits. Sodium salts of anticoagulants are
available as concentrates to be mixed with water, making
a liquid bait (Fig. 8). Although mice require little or
no water to survive, they will readily drink it when
available. Water baits can be an effective supplement to
other control measures where water is scarce. They are
particularly useful in grain storage structures,
warehouses, and other such locations. Rodents are more
easily able to detect anticoagulants in water baits than
in food baits; therefore, up to 5% sugar is sometimes
added to liquid baits to increase rodents’ acceptance of
the bait solution. Since water is attractive to most
animals, use water baits in ways that prevent nontarget
animals from drinking them. Bait Stations
than dry baits. Sodium salts of anticoagulants are
available as concentrates to be mixed with water, making
a liquid bait (Fig. 8). Although mice require little or
no water to survive, they will readily drink it when
available. Water baits can be an effective supplement to
other control measures where water is scarce. They are
particularly useful in grain storage structures,
warehouses, and other such locations. Rodents are more
easily able to detect anticoagulants in water baits than
in food baits; therefore, up to 5% sugar is sometimes
added to liquid baits to increase rodents’ acceptance of
the bait solution. Since water is attractive to most
animals, use water baits in ways that prevent nontarget
animals from drinking them. Bait Stations
 Bait
stations (bait boxes) may increase both the
effectiveness and safety of rodenticides. They came into
general use after the development of the
first-generation anticoagulants, which require that a
continuous supply of bait be made available to rodents.
Bait stations are useful because they: Bait
stations (bait boxes) may increase both the
effectiveness and safety of rodenticides. They came into
general use after the development of the
first-generation anticoagulants, which require that a
continuous supply of bait be made available to rodents.
Bait stations are useful because they:
— protect bait from
moisture and dust; —provide a protected place for
rodents to feed, allowing them to feel more secure. This
is an important advantage when baiting mice, which
apparently like to spend time feeding inside such bait
boxes; —keep other animals (pets, livestock, desirable
wildlife) and children away from hazardous bait; —allow
placement of bait in locations where it would otherwise
be difficult because of weather or potential hazards to
nontarget animals; —help prevent the accidental spilling
of bait; —allow easy inspection of bait to see if
rodents are feeding on it.
Kinds of Bait Stations.
Bait stations can contain solid baits (food baits),
liquid baits, or both. Bait boxes can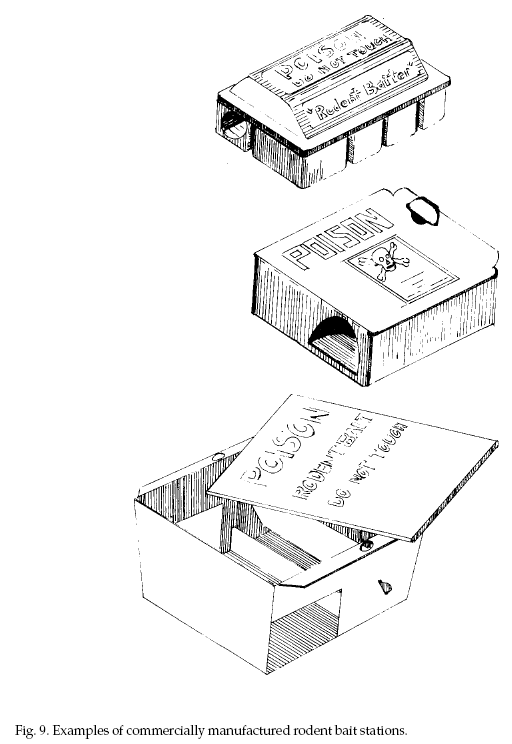 be purchased from commercial suppliers or made at home.
Manufactured bait boxes made of plastic, cardboard, or
metal are sold to pest control companies and to the
public (Fig. 9) in sizes for rats or mice. Some farm
supply and agricultural chemical supply stores have them
in stock or can order them. Recent research suggests
mice may prefer to feed in cardboard bait stations
rather than plastic ones.
be purchased from commercial suppliers or made at home.
Manufactured bait boxes made of plastic, cardboard, or
metal are sold to pest control companies and to the
public (Fig. 9) in sizes for rats or mice. Some farm
supply and agricultural chemical supply stores have them
in stock or can order them. Recent research suggests
mice may prefer to feed in cardboard bait stations
rather than plastic ones.
Bait boxes can be built
from scrap materials, and homemade stations can be
designed to fit individual needs. Make them out of
sturdy materials so they cannot be easily knocked out of
placeor damaged. Where children, pets, or livestock are
present, be careful to construct the stations so that
the bait is accessible only to rodents. Locks, seals, or
concealed latches are often used to make bait boxes more
tamperproof. Clearly label all bait boxes or stations
with “Poison” or “Rodent Bait — Do Not Touch,” or with a
similar warning. Some rodenticides or situations may
require use of tamper-resistance bait stations. If so,
use only bait boxes or stations which are so designated,
and also be sure to secure them to buildings by nailing
or gluing them to walls or floors in a way that will not
permit a person or animal to knock them over or shake
the bait out.
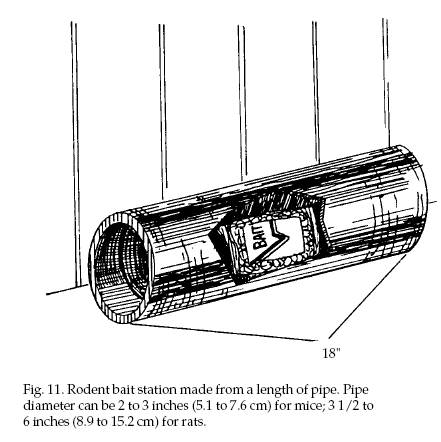
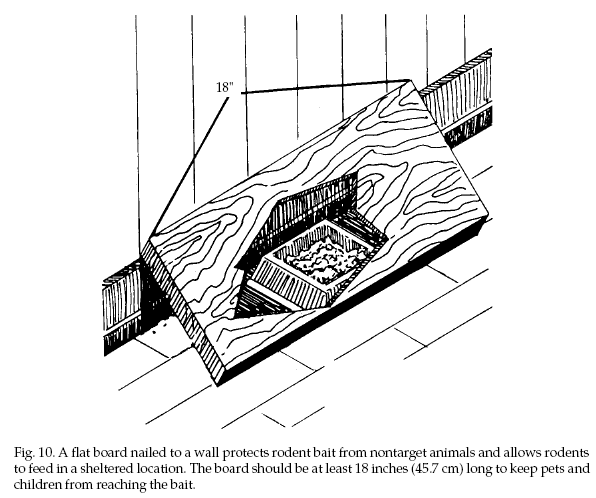
Bait Station Design.
Bait stations should be large enough to allow several
rodents to feed at once. They can be as simple as a flat
board nailed at an angle to the bottom of a wall (Fig.
10), or a length of pipe into which bait can be placed
(Fig. 11). More elaborate stations are completely
enclosed and can contain
liquid as well as solid rodent baits (Fig. 12). A hinged
lid with a child-proof latch can be used for convenience
in inspecting permanent stations.
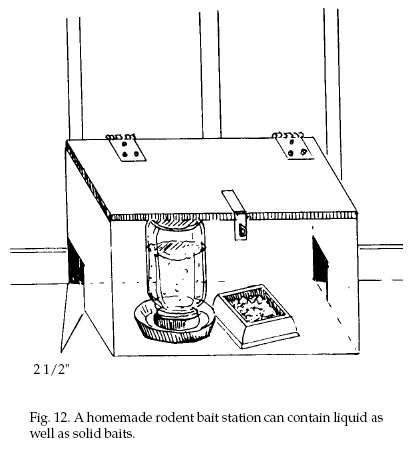
Bait stations for mice
should have at least two openings approximately 1 inch
(2.5 cm) in diameter. Locate the two holes on opposite
sides of the station so that mice can see an alternate
escape route as they enter the station. Bait Station
Maintenance. Baits must be fresh and of high quality.
Mice may reject spoiled or stale foods. Provide enough
fresh bait to allow rodents to eat all they want. When
using rodenticides designed for continuous bait
application (such as anticoagulants), bait station
maintenance is essential to a successful baiting effort.
When bait boxes are first put out, check them daily and
add fresh bait as needed. After a short time, as rodent
numbers and feeding decline, check the boxes once every
2 to 4 weeks. If the bait becomes moldy, musty, soiled,
or insect-infested, empty the box and clean it, and then
refill it with fresh bait. Dispose of spoiled or uneaten
bait in accordance with the label. Follow all label
directions for the product you are using.
Placement of Bait
Stations. House mice are active in a small area and lack
notable food preferences. Therefore, proper placement of
baits or bait stations is often more important than the
type of bait used. Mice will not visit bait stations,
regardless of their contents, if not conveniently
located in areas where they are active.
Where possible, place bait
between the rodents’ source of shelter and their food
supply. Put bait boxes near rodent burrows, against
walls or along travel routes. Where mice are living in
sacked or boxed feed on pallets, baits or traps may have
to be placed on top of stacks or wedged in gaps within
the stacks. In such situations, this “three dimensional”
bait placement is important to obtain good control.
Caution should be used in selecting control methods in
such situations. Do not use baits that will contaminate
foodstuffs. For safety, it may not be wise to use toxic
baits in the vicinity of certain foodstuffs. Traps or
glue boards may be used instead.
On farmsteads, bait
station placement depends on building design and use.
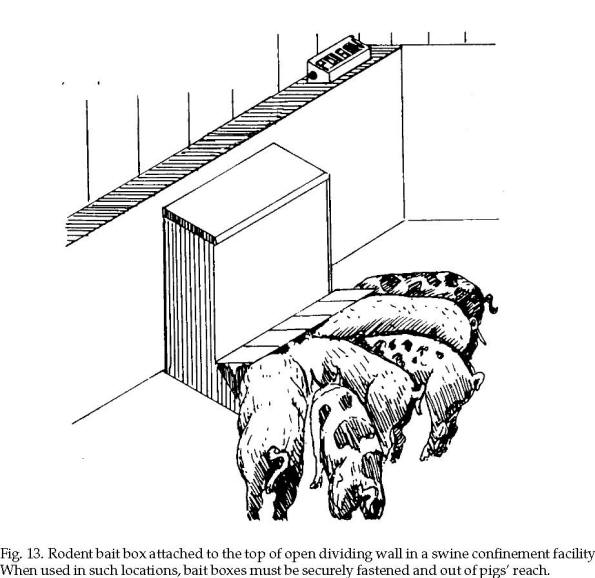
For example, in swine
confinement buildings it may be possible to attach bait
boxes to wall ledges or the top of pen dividing walls.
Bait boxes may be placed in attics or along floors or
alleys where rodents are active (Fig. 13). Rodent tracks
visible on dusty surfaces and their droppings often give
clues to where they are active.
Never place bait stations
where livestock, pets, or other animals can knock them
over. Spilled bait may be a potential hazard,
particularly to smaller animals.
Where buildings are not
rodent-proof, permanent bait stations can be placed
inside buildings, along the outside of building
foundations, or around the perimeter. Bait stations will
help keep rodent numbers at a low level when maintained
regularly with fresh anticoagulant bait. Rodents moving
in from nearby areas will be controlled before they can
reproduce and cause serious damage.
Tracking Powders. Toxic
dusts or powders have been successfully used for many
years to control mice and rats. When mice walk over a
patch of toxic powder, they pick some of it up on their
feet and fur and later ingest it while grooming.
Tracking powders are useful in controlling mice where
food is plentiful and good bait acceptance is difficult
to achieve. Mice are more likely to ingest a lethal
amount of a poorly accepted toxicant applied by this
method than if it is mixed into a bait material. There
is little likelihood of toxicant shyness developing when
using tracking powders.
Because the amount of
material a mouse may ingest while grooming is small, the
concentration of active ingredient in tracking powders
is considerably higher than in food baits that utilize
the same toxicant. Therefore, these materials can be
more hazardous than food baits. For the most part,
tracking powders are used by professional pest control
operators and others trained in rodent control. Tracking
powders containing either zinc phosphide or
anticoagulants are commercially available, although some
are Restricted Use Pesticides.
Place tracking powders
along runways, in walls, behind boards along walls, or
on the floor of bait stations. Placement can be aided by
using various types of sifters, shakers, or blowers.
Dampness may cause the powder to cake and lessen its
effectiveness. Care must be taken to place tracking
powders only where they cannot contaminate food or
animal feed, or where nontarget animals cannot come into
contact with them. Do not place tracking powders where
mice can track the material onto food intended for use
by humans or domestic animals. Tracking powders are not
generally recommended for use in and around homes
because of the potential hazards to children and pets.
Where possible, remove tracking powder after the rodent
control program is completed. Tracking powders used in
conjunction with baiting can provide very effective
mouse control.
Fumigants
Fumigants (toxic gases)
are most commonly used to control mice in structures or
containers such as feed bins, railway cars, or other
enclosed areas. Aluminum phosphide, chloropicrin, and
methyl bromide are currently registered for this
purpose. Some fumigant materials are registered for use
in rodent burrows; however, house mouse burrows cannot
be fumigated efficiently or economically because they
are small and often difficult to find. Generally,
control of house mice by fumigation is only practical
and cost-effective in a very limited number of
situations. Fumigants are hazardous materials and should
be applied only by persons well trained in their use and
who possess the necessary safety equipment.
Trapping
Trapping can be an
effective method of controlling mice, but it requires
more labor than most other methods. Trapping is
recommended where poisons seem inadvisable. It is the
preferred method to try first in homes, garages, and
other small structures where there may be only a few
mice present.
Trapping has several
advantages: (1) it does not rely on inherently hazardous
rodenticides; (2) it permits the user to view his or her
success; and (3) it allows for disposal of the mice,
thereby eliminating odor problems from decomposing
carcasses that may remain when poisoning is done within
buildings.
The simple, inexpensive,
wood-based snap trap is available in most hardware and
farm supply stores. Traps should be baited with a small
piece of nutmeat, chocolate candy, dried fruit, or bacon
tied securely to the trigger. Peanut butter or
marshmallows also may be used as bait. Because mice are
always in search of nesting materials, a small cotton
ball will also work as a bait when attached securely to
the trigger. Food baits that become stale lose their
effectiveness.
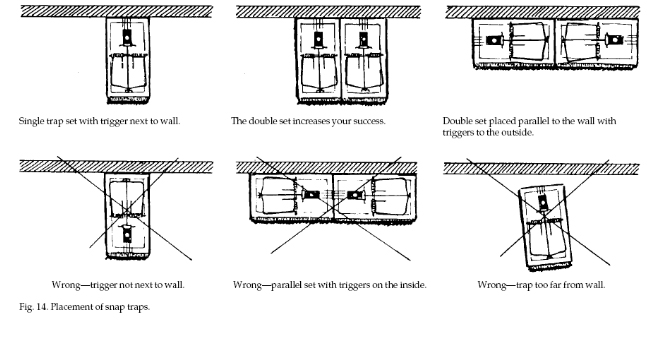
Set traps close to walls,
behind objects, in dark places, and in locations where
mouse activity is seen. Place the traps so that when
mice follow their natural course of travel (usually
close to a wall) they will pass directly over the
trigger (Fig. 14). Set traps so that the trigger is
sensitive and will spring easily. Effectiveness can be
increased by enlarging the trigger. Attach a square of
cardboard, metal, or screen wire that fits just inside
the wire deadfall (Fig. 15).
Use enough traps to make
the campaign short and decisive. Mice seldom venture far
from their shelter and food supply, so traps should be
spaced no more than about 6 feet (1.8 m) apart in areas
where mice are active. Although mice are not nearly as
afraid of new objects as rats are, leaving the traps
baited but unset until the bait is taken at least once
will reduce the chance of mice escaping the trap and
becoming trap-shy.
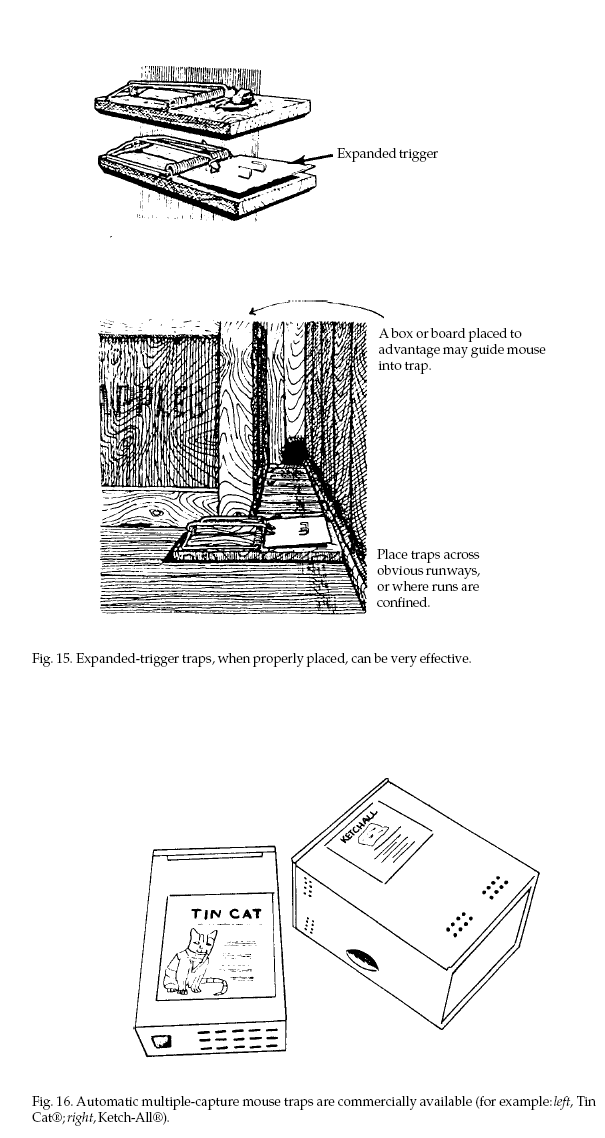
Multiple-capture
(automatic) mouse traps such as the Ketch-All® and
Victor Tin Cat® (Fig. 16) are available from some
hardware and farm supply stores as well as from pest
control equipment distributors. These traps work on the
principle that mice enter small holes without
hesitation. The Ketch-All® has a wind-up spring that
powers a rotating mechanism. When triggered, the
mechanism entraps mice in a holding compartment. The Tin
Cat® has one-way doors that mice cannot exit. Such traps
may catch many mice in a single setting, but should be
checked and emptied periodically so that mice do not die
of starvation or exposure in the traps.
Various types of box-type
traps (Sherman-type and others) that capture one mouse
at a setting are used primarily for research purposes.
The desire to “build a better mousetrap” keeps a variety
of traps of variable effectiveness coming and going on
the retail market.
Keep traps reasonably
clean and in good working condition. They can be cleaned
with a hot detergent solution and a stiff brush. Human
and dead-mouse odors on traps are not known to reduce
trapping success.
An alternative to traps
are glue boards, which catch and hold mice attempting to
cross them, much the way flypaper catches flies. Place
glue boards wherever mice travel—along walls or in
established runways. Do not use glue boards where
children, pets, or desirable wildlife can contact them.
Glue boards lose their effectiveness in dusty areas
unless covered, and temperature extremes may affect the
tackiness of some glues. They are considered less
effective for capturing rats than for mice. Glue boards
can be purchased ready-to-use, or they can be made.
Euthanize live, trapped
rodents by carbon dioxide asphyxiation or use a stick to
kill them with sharp blows to the base of the skull. For
further information on glue boards, see the section
Supplies and Materials.
Other Methods
Some dogs and cats will
catch and kill mice and rats. There are few situations,
however, in which they will do so sufficiently to
control rodent populations. Around most structures, mice
can find many places to hide and rear their young out of
the reach of such predators. Cats probably cannot
eliminate existing mouse populations, but in some
situations they may be able to prevent reinfestations
once mice have been controlled. Farm cats, if sufficient
in number and supplementally fed, may serve this
function.
In urban and suburban
areas, it is not uncommon to find rodents living in
close association with cats and dogs, relying on cat and
dog food for nourishment. Mice frequently live beneath
dog houses and soon learn they can feed on their food
when they are absent or asleep.
Economics of Damage and Control
Accurate data on mouse
damage, control, and their cost are difficult to obtain.
Estimates of losses of foodstuffs, structural damage,
and the amount of labor and materials expended to
control mice are usually only educated guesses.
In one survey of corn in a
midwestern state, 76% of about 1,000 grain samples were
contaminated with rodent droppings. Mouse droppings
outnumbered rat droppings twelve to one. A house mouse
produces about 36,000 droppings in a year’s time. Mouse
infestations are so widespread that droppings and hairs
often end up in many types of food commodities intended
for human use. Certain levels of rodent contamination
are grounds for condemning food commodities.
Structural damage caused
by rodents can be expensive. In recent years, the trend
toward use of insulated confinement facilities to raise
swine in the northern Great Plains has led to an
increased amount of rodent damage. Mice, in particular,
are very destructive to rigid foam, fiberglass batt, and
other types of insulation in walls and attics of such
facilities. In one small swine finishing building near
Lincoln, Nebraska, rodent damage required the producer
to spend $5,000 in repairs to the facility only 3 years
after initial construction.
Acknowledgments
I thank Rex E. Marsh for
reviewing a previous version of this chapter and
providing many helpful comments. Portions of the
recommendations on toxicant use are taken directly from
his chapter Roof Rats in this manual. Other material
contained in this chapter is derived from Brooks (1973),
Marsh and Howard (1981), and Pratt et al. (1977), among
other sources.
Figure 1 is from Schwartz
and Schwartz (1981).
Figures 2, 5, and 15 were
adapted from Pratt et al. (1977) by Jill Sack Johnson.
Figures 3 and 14 were
adapted from Howard and Marsh (1981) by Jill Sack
Johnson.
Figure 4 from Hygnstrom
and Virchow (1992).
Figures 6, 7, and 8 were
developed by Jill Sack Johnson.
Figures 9, 12, and 13 are
by Frances I. Gould, University of Nebraska-Lincoln,
Cooperative Extension.
Figures 10 and 11 were
adapted from Pratt et al. (1977) by Frances I. Gould.
For
Additional Information
Berry, R. J. 1981. Town
mouse, country mouse: adaptation and adaptability in Mus
domesticus (M. musculus d). Mammal Rev. 11:91-136.
Berry, R. J., ed. 1981.
Biology of the house mouse. Symp. Zool. Soc. London, No.
47. 715 pp.
Bohills, S. T., A. P.
Meehan, and S. P. Leonard. 1982. Advantages of bait
boxes in house mouse control. International Pest Control
24(2):34, 35, 37.
Bronson, F. H. 1979. The
reproductive ecology of the house mouse. Quarterly Rev.
Biol. 54(3):265-299.
Brooks, J. E. 1973. A
review of commensal rodents and their control. CRC
Critical Rev. Environ. Control 3:405-453.
Burt, W. H., and R. P.
Grossenheider. 1976. A field guide to the mammals, 3rd
ed. Houghton Mifflin Co., Boston. 289 pp.
Chitty, D., and H. N.
Southern. 1954. Control of rats and mice, vol. 1-3.
Clarendon Press, Oxford.
Corrigan, R. M., C. A.
Towell, and R. E. Williams. 1992. Development of rodent
control technology for confined swine facilities. Proc.
Vertebr. Pest Conf. 15:280-285.
Crowcroft, P., and J. N.
R. Jeffers. 1961. Variability in the behavior of wild
house mice (Mus musculus L.) towards live traps. Proc.
Zool. Soc. London 137:573-582.
Davis, D. E. 1981.
Environmental control of rodents. Pages 493-498 in D.
Pimentel, ed. CRC handbook of pest management in
agriculture, vol. 1. CRC Press, Inc., Boca Raton,
Florida.
Fitzwater, W. D. 1982.
Bird limes and rat glues — sticky situations. Proc.
Vertebr. Pest Conf. 10:17-20.
Frantz, S. C., and D. E.
Davis. 1991. Bionomics and integrated pest management of
commensal rodents. Pages 243-313 in J. R. Gorham, ed.
Ecology and management of food-industry pests. Food Drug
Admin. Tech. Bull. 4, Assoc. Official Analytical Chem.
Arlington, VA.
Haines, H., and K.
Schmidt-Neilsen. 1967. Water deprivation in wild house
mice. Physiol. Zool. 40:424-431.
Howard, W. E., and R. E.
Marsh. 1981. The rat: its biology and control. Div.
Agric. Sci. Univ. California, Leaflet 2896 (revised). 30
pp.
Humphries, R. E., A. P.
Meehan, and R. M. Sibly. 1992. The characteristics and
history of behavioural resistance in inner-city house
mice (Mus domesticus) in the U.K. Proc. Vertebr. Pest
Conf. 15:161-164.
Hygnstrom, S. E., and D.
R. Virchow. 1992. Controlling house mice. Univ. Nebraska
Coop. Ext. NebGuide G92-1105-A. 4 pp.
Jackson, W. B. 1990. Rats
and mice. Pages 9-85 in A. Mallis, ed. Handbook of pest
control. Franzak and Foster Co., Cleveland, OH.
Kaukeinen, D. E. 1982. A
review of the secondary poisoning hazard potential to
wildlife from the use of anticoagulant rodenticides.
Proc. Vertebr. Pest Conf. 10:151-158.
Kaukeinen, D. E. 1984.
Resistance: what we need to know. Pest Manage.
3(3):26-30.
Knote, C. E. 1988.
Stopping house mice building infestations through
exterior control. Proc. Vertebr. Pest Conf. 13:107-111.
Labov, J. B. 1981. Male
social status, physiology, and ability to block
pregnancies in female house mice (Mus musculus). Behav.
Ecol. Sociobiol. 8:287-291.
Marsh, R. E., and W. E.
Howard. 1981. The house mouse: its biology and control.
Div. Agric. Sci. Univ. California, Leaflet 2945
(revised). 30 pp.
Meehan, A. P. 1984. Rats
and mice: their biology and control. Rentokil Ltd., E.
Grinstead, U.K. 383 pp.
Morris, K. D., and D. E.
Kaukeinen. 1988. Comparative evaluation of tamper-proof
mouse bait stations. Proc. Vertebr. Pest Conf.
13:101-106.
Petras, M. L., and J. C.
Topping. 1981. Studies of natural populations of Mus.
VI. Sizes of populations inhabiting corn cribs in
southwestern Ontario. J. Mammal. 62:146-153.
Pratt, H. D., B. F.
Bjornson, and K. S. Littig. 1977. Control of domestic
rats and mice. Public Health Serv., US Dep. Health, Educ.
Welfare, Pub. No. (CDC) 77-841. 47 pp.
Robbins, R. J. 1980.
Taste-aversion learning and its implications for rodent
control. Proc. Vertebr. Pest Conf. 9:114-121.
Rowe, F. P. 1966. Economic
importance of the house mouse (Mus musculus L.). World
Health Organiz., Vector Control, Pub. 66.217, paper
1.4:21-26.
Schwartz, C. W., and E. R.
Schwartz. 1981. House mouse. Pages 248-252 in The wild
mammals of Missouri, rev. ed. Univ. Missouri Press,
Columbia. 356 pp.
Shenker, A. M. 1973. The
house mouse in London. Mammal Rev. 3:64-69.
Weber, W. J. 1982.
Diseases transmitted by rats and mice. Thomson Pub.
Fresno, California. 182 pp.
Editors
Scott E. Hygnstrom Robert
M. Timm Gary E. Larson
PREVENTION AND CONTROL OF
WILDLIFE DAMAGE — 1994
Cooperative Extension
Division Institute of Agriculture and Natural Resources
University of Nebraska -Lincoln
United States Department
of Agriculture Animal and Plant Health Inspection
Service Animal Damage Control
Great Plains Agricultural
Council Wildlife Committee
Special
thanks to:
Clemson University
|