|
|
|
|
 |
RODENTS: Polynesian Rats |
|
|

Fig. 1. Polynesian rat,
Rattus exulans
- Exclusion
Not practical for Hawaiian sugarcane fields.
- Cultural Methods
Synchronize planting and harvesting of large blocks
of fields.
Eliminate or modify noncrop vegetation adjacent to
sugarcane fields.
Develop potential resistant sugarcane varieties.
- Repellents
None are registered.
- Toxicants
Zinc phosphide.
- Fumigants
Not practical in and around sugarcane fields.
- Trapping
Not practical in and around sugarcane fields.
- Shooting
Not practical.
- Biological Control
Not effective.
Identification
The Polynesian rat (Rattus
exulans) is smaller than either the Norway rat (R.
norvegicus) or the roof rat (R. rattus). Polynesian rats
have slender bodies, pointed snouts, large ears, and
relatively small, delicate feet. A ruddy brown back
contrasts with a whitish belly. Mature individuals are
4.5 to 6 inches long (11.5 to 15.0 cm) from the tip of
the nose to the base of the tail and weigh 1.5 to 3
ounces (40 to 80 g). The tail has prominent fine scaly
rings and is about the same length as the head and body.
Female Polynesian rats have 8 nipples, compared to 10
and 12 nipples normally found on roof rats and Norway
rats, respectively.
Range
Polynesian rats are native
to Southeast Asia but have dispersed with humans across
the central and western Pacific. Today, these rodents
inhabit almost every Pacific island within 30o of the
equator. They occur from the Asiatic mainland south to
New Guinea and New Zealand, and east to the Hawaiian
Islands and Easter Island. Polynesian rats accompanied
early Polynesian immigrants to Hawaii and today occur on
every major island of the archipelago. The Polynesian
rat is not present in the mainland United States.
Habitat
In Hawaii, Polynesian rats
are most common below 2,500 feet (750 m) elevation,
although individuals have been captured at an elevation
of 4,900 feet (1,500 m) on Mauna Loa on the island of
Hawaii and 9,700 feet (2,950 m) on the rim of Haleakala
Crater on Maui. Polynesian rats prefer areas with good
ground cover on well-drained soil. Throughout much of
their range, Polynesian rats live in close association
with humans. In Hawaii, however, Polynesian rats are not
a commensal pest, but rather favor wild lowland habitats
such as wooded and grassy gulches, fields, and waste
areas. They reach their highest densities on
agricultural lands such as sugarcane fields and
abandoned pineapple fields.
Food Habits
Polynesian rats eat a wide
variety of foods, including broadleaf plants, grasses,
fruits, seeds, and animal matter. They prefer fleshy
fruits such as melastoma (Melastoma malabathricum),
passion fruit (Passiflora spp.), guava (Psidium spp.),
thimbleberry (Rubus rosaefolius), and popolo (Solanum
nodiflorum). In sugarcane fields, sugarcane comprises
about 70% of their diet by volume, while in surrounding
noncrop gulches, it comprises about 20% to 50%. Rats
cannot subsist on sugarcane alone. They need additional
protein, such as earthworms, spiders, amphipods,
insects, and eggs and young of ground-nesting birds.
General
Biology, Reproduction, and Behavior
Reproduction varies among
geographic areas and is influenced by weather,
availability of food, and other factors. Reproductive
activity of Polynesian rats on Oahu reaches a peak in
late summer and ceases in mid to late winter. Polynesian
rats on Kure Atoll in northwestern Hawaii produce most
litters from May through August. On the windward side of
the island of Hawaii, Polynesian rats breed throughout
the year, with peak reproduction occurring in the summer
and early fall. Females have an average of 4 litters per
year, with a range of 3 to 6 and an average of 4 young
per litter. The minimum gestation period for captive
rats is 23 days, with lactation prolonging gestation by
3 to 7 days. In captivity, newborns open their eyes
about 2 weeks after birth and are weaned when about 3
weeks old. Captive-bred individuals reach reproductive
maturity when they are 60 to 70 days old and weigh about
1.5 ounces (40 g). The life expectancy of wild rats is
less than 1 year.
Hawaii is one of the few
areas in the world where sugarcane is grown as a 2-to
3-year crop. Most rats living in cane fields either die
or migrate to surrounding areas during harvest, and
populations do not rebuild until the second half of the
crop cycle. During much of the first year, the sugarcane
stalks stand erect, the crop canopy is open, and most
fields have little ground cover. Some rats from adjacent
waste areas forage along the periphery of young
sugarcane fields, but few venture into the interior
until the sugarcane is 8 to 12 months of age. At this
time the sugarcane stalks fall over and dead leaves
accumulate. The resulting thatch layer is rich in
invertebrate food and provides protective cover in
fields where rats establish dens.
Movements and home ranges
in sugarcane fields vary depending on population
density, crop age, and other factors. Polynesian rats
are nocturnal and are relatively sedentary. Males travel
farther than females, but the home ranges of both sexes
decrease as the sugarcane matures. Individuals typically
stray less than 100 to 165 feet (30 to 50 m) from their
burrows.
Population
Changes
Roof rats, Norway rats,
and Polynesian rats coexist throughout much of the
Pacific basin. It is not known how much, if any,
interspecific competition exists. After the arrival of
Norway rats, roof rats, and house mice (Mus musculus) in
New Zealand, populations of Polynesian rats declined.
Today, they are very rare on the two main islands. It is
not clear whether a similar decline occurred in Hawaii,
but if so, Polynesian rats have adjusted. Today, they
are the most abundant lowland rat in many parts of the
state.
In Hawaii, roof rats,
Norway rats, and Polynesian rats often occur in the same
sugarcane fields. Only the latter two are major pests in
sugarcane, with roof rats occurring mostly near field
edges. Since the late 1960s Norway rats have increased
their abundance relative to the other two species in
Hawaiian sugarcane fields and are now the species of
primary concern to the Hawaiian sugarcane industry.
Polynesian rats, however, are still locally abundant in
many fields.
Damage
and Damage Identification
Polynesian rats are a
major agricultural pest throughout Southeast Asia and
the Pacific region. Crops damaged by this species
include rice, maize, sugarcane, coconut, cacao,
pineapple, and root crops. In the United States,
sugarcane is the only crop of economic concern damaged
by Polynesian rats. The most severe damage is to
unirrigated sugarcane on the windward side of the
islands of Hawaii and Kauai. Here, rats find excellent
habitat in the lush vegetation of noncrop lands adjacent
to sugarcane fields.
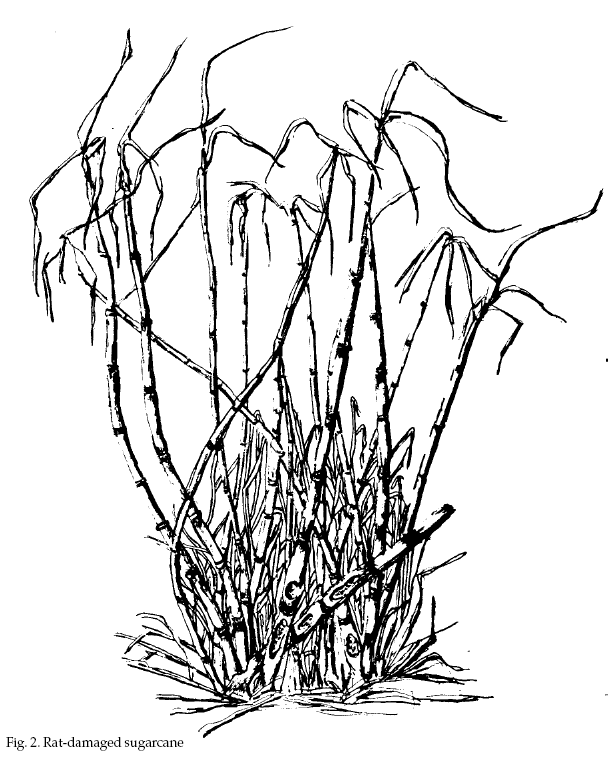
Rat damage to Hawaiian
sugarcane is negligible until the crop is 14 to 15
months old, after which it increases substantially and
progressively until harvest. Damage caused by roof rats,
Norway rats, and Polynesian rats is very similar. All
three species chew on the internodes of growing stalks.
Injury ranges from barely perceptible nicks in the outer
rind to neatly chiseled canoe-shaped cavities. Small
chips usually are evident on the ground where rats have
fed. Rat depredation can be distinguished easily from
that of feral pigs (Sus scrofa). Pigs chew on the entire
stalk, leaving it with a shredded appearance. Trampled
vegetation is further evidence of pig activity.
Legal Status
Rats are an exotic species
in Hawaii and are not protected by law. They may be
controlled by any method consistent with state and
federal laws and regulations.
Damage
Prevention and Control Methods
Exclusion
Electric fences and
physical barriers have been used to prevent rats from
entering experimental farm plots. It is questionable,
however, whether current fencing designs and exclusion
techniques are practical for Hawaiian sugarcane fields.
Cultural Methods
Advancing harvest from the
usual 22to 24-month schedule would reduce losses.
Adoption of a shorter crop cycle, however, would
increase planting and harvesting costs and probably
would not be feasible considering current economic
conditions. Synchronized planting and harvesting of
adjacent fields might reduce movements of rats from
recently harvested fields into younger fields.
Modification or elimination of noncrop vegetation
adjacent to sugarcane fields would help reduce invasion
from surrounding areas. Cattle grazing or commercial
production of trees for energy or timber might reduce
the vegetative understory in such areas. Herbicide use
probably is not economical or environmentally desirable.
Development of sugarcane
varieties that are less susceptible to damage by rats is
a promising avenue for research. Possible selection
criteria include rind hardness, stalk diameter, degree
and time of lodging, resistance to souring, and
potential for compensatory growth.
Repellents
None are registered.
Toxicants
Zinc phosphide is the only
toxicant registered in the United States for rat control
in sugarcane. Baits are formulated either as pellets or
on oats and usually are broadcast by fixed-wing aircraft
at the rate of 5 pounds per acre (5.6 kg/ha). A maximum
of four applications and 20 pounds per acre (22.4 kg/ha)
may be applied per crop cycle.
Zinc phosphide baits in
Hawaii are most effective against Polynesian rats and
least effective against Norway rats. Because the
relative abundances of the two species vary
substantially from field to field and may shift as the
crop matures, the efficacy of zinc phosphide baits also
varies. Where Norway rat populations increase during the
second year of the crop cycle, zinc phosphide baits
become progressively less effective.
Fumigants
None are registered for
the control of Polynesian rats in Hawaii.
Trapping
Polynesian rats can be
captured easily with coconut bait and standard snap
traps, modified wire-cage Japanese live traps, or other
appropriate traps.
However, trapping in
sugarcane fields is extremely labor intensive and is not
practical for control purposes. Plantation personnel
took an average of 141,000 rats annually from sugarcane
fields on the island of Hawaii during the early 1900s,
but with no apparent effect either on rat populations or
on sugarcane damage (Pemberton 1925).
Shooting
This is not a practical
form of population control.
Biological
Control
In 1883, the Indian
mongoose (Herpestes auropunctatus) was introduced into
Hawaii from the West Indies to help control rats on
sugarcane plantations, and today they are common on all
the major islands except Kauai. Although mongooses are
diurnal and rats are nocturnal, rodents comprise the
major portion of the mongoose’s diet in and around
sugarcane fields. Pemberton (1925) found parts of
rodents in 88% of 356 mongoose pellets collected in
sugarcane fields, with 52% of all samples containing
nothing but rodent parts. Kami (1964) reported that 72%
of 393 mongoose scats collected along dirt roads
adjacent to cane fields contained rodent pelage and
bones. However, rats reproduce rapidly and continue to
thrive and cause major economic damage in Hawaii. Not
only has the introduction of the mongoose failed to
control rat populations, but it has resulted in
unforeseen ecological effects. Mongoose predation has
been implicated in the decline of the Hawaiian goose (Nesochen
sandvicensis), Newell’s shearwater (Puffinus newelli),
and other ground-nesting birds in Hawaii. If rabies ever
becomes established in Hawaii, the mongoose is likely to
become a public health concern.
Between 1958 and 1961,
barn owls (Tyto alba) also were introduced into the
state to help control rodent agricultural pests. This
species and the native short-eared owl (Asio flammeus)
subsist in Hawaii in large part on rodents. Although
raptors sometimes are attracted to rats fleeing recently
harvested sugarcane fields, heavy thatch prevents their
foraging in maturing sugarcane fields.
Dogs have also been used
to control rats in harvested sugarcane fields (Pemberton
1925, Doty 1945), but controls applied after harvest are
likely to have little effect on damage or yields.
Economics of Damage and
Control In addition to direct losses, secondary
infections of stalks by insects and pathogens result in
additional losses of stalks and deterioration of cane
juice. The economic impact of these losses fluctuates
from year to year, largely dependent on the prevailing
price of sugar. In 1980, when the average price of raw
sugar was at a 50-year high, the Hawaiian sugarcane
industry may have lost $20 million. Current losses are
conservatively estimated to be greater than $6 million
annually (A. Ota, Hawaiian Sugar Planters’ Association,
pers. commun.).
Aerially broadcasting 5
pounds of zinc phosphide-treated oats to 1 acre (5.6
kg/ha) of sugarcane costs approximately $4.99, including
$3.50 for bait, $1.33 for the airplane, fuel, and pilot,
and $0.16 for labor, transportation of materials,
administrative overhead, and other expenses. The
registration label calls for four applications during
the crop cycle, which would cost about $20.00 per acre
($50.00/ha). Studies have indicated that applications of
zinc phosphide reduce damage in Hawaiian sugarcane
fields by as much as 30% to 45%. Thus, four applications
of zinc phosphide would result in savings of $120 to
$185 per acre ($296 to $475/ ha), or a return of $6.00
to $9.00 for every $1.00 spent applying bait. This
assumes a potential yield of 10 tons per acre (22.5 mt/ha)
without applying controls, a farm price of $368 per ton
($409/mt), and a 10% decrease in yield due to rat
damage. The benefits of using zinc phosphide are less in
fields with lower damage.
Acknowledgments
D. Fellows, L. Fiedler, A.
Koehler, and R. Sugihara reviewed earlier drafts of this
chapter.
D. Steffen sketched the
line drawings.
For Additional Information
Doty, R. E. 1945. Rat control on Hawaiian sugarcane
plantations. Hawaiian Planters’ Rec. 49:72-241.
Fellows, D. P. and R. T.
Sugihara. 1977. Food habits of Norway and Polynesian
rats in Hawaiian sugarcane fields. Hawaiian Planters’
Rec. 59:67-86.
Hirata, D. N. 1977.
Species composition of rats on Mauna Kea sugar company
from 1967 through 1976. Hawaiian Sugar Technol. 1977
Rep. pp. 91-94.
Hood, G. A., R. D. Nass,
and G. D. Lindsey. 1970. The rat in Hawaiian sugarcane.
Proc. Vertebr. Pest Conf. 4:34-37.
Hood, G. A., R. D. Nass,
G. D. Lindsey, and D. N. Hirata. 1971. Distribution and
accumulation of rat damage in Hawaiian sugarcane. J.
Wildl. Manage. 35:613-618.
Kami, H. T. 1964. Foods of
the mongoose in the Hamakua District, Hawaii. Zoonoses
Res. 3:165-170.
Nass, R. D., G. A. Hod,
and G. D. Lindsey. 1971. Fate of Polynesian rats in
Hawaiian sugarcane fields during harvest. J. Wildl.
Manage. 35:353-356.
Pemberton, C. E. 1925. The
field rat in Hawaii and its control. Hawaiian Sugar
Planters’ Assoc. Exp. Stn. Bull. No. 17. 46 pp.
Sugihara, R. T., L. F.
Pank, D. P. Fellows, D. N. Hirata, R. S. Stott, H. W.
Hilton, and H. Kaya. 1977. Noncrop habitat manipulation
as a means of controlling rats and reducing damage to
sugarcane. Hawaiian Sugar Technol. Rep. pp. 83-90.
Tobin, M. E., and R. T.
Sugihara. 1992. Abundance and habitat relationships of
rats in Hawaiian sugarcane fields. J. Wildl. Manage.
56:815-821.
Tobin, M. E., and R. T.
Sugihara, and A. K. Ota. 1990. Rodent damage to Hawaiian
sugarcane. Proc. Vertebr. Pest Conf. 14:120-123.
Tomich, P. Q. 1970.
Movement patterns of field rodents in Hawaii. Pacific
Sci. 24:195-234.
Tomich, P. Q. 1986.
Mammals in Hawaii, rev. ed. Bishop Museum Press,
Honolulu. 375 pp.
van Riper, S. G., and C.
van Riper III. 1982. A field guide to the mammals in
Hawaii. The Oriental Publ. Co. Honolulu. 68 pp.
Editors
Scott E. Hygnstrom, Robert
M. Timm, Gary E. Larson
PREVENTION AND CONTROL OF
WILDLIFE DAMAGE — 1994
Cooperative Extension
Division Institute of Agriculture and Natural Resources
University of Nebraska -Lincoln
United States Department
of Agriculture Animal and Plant Health Inspection
Service Animal Damage Control
Great Plains Agricultural
Council Wildlife Committee
Special
thanks to:
Clemson University
|