|
|
|
|
 |
OTHER MAMMALS: Shrews |
|
|
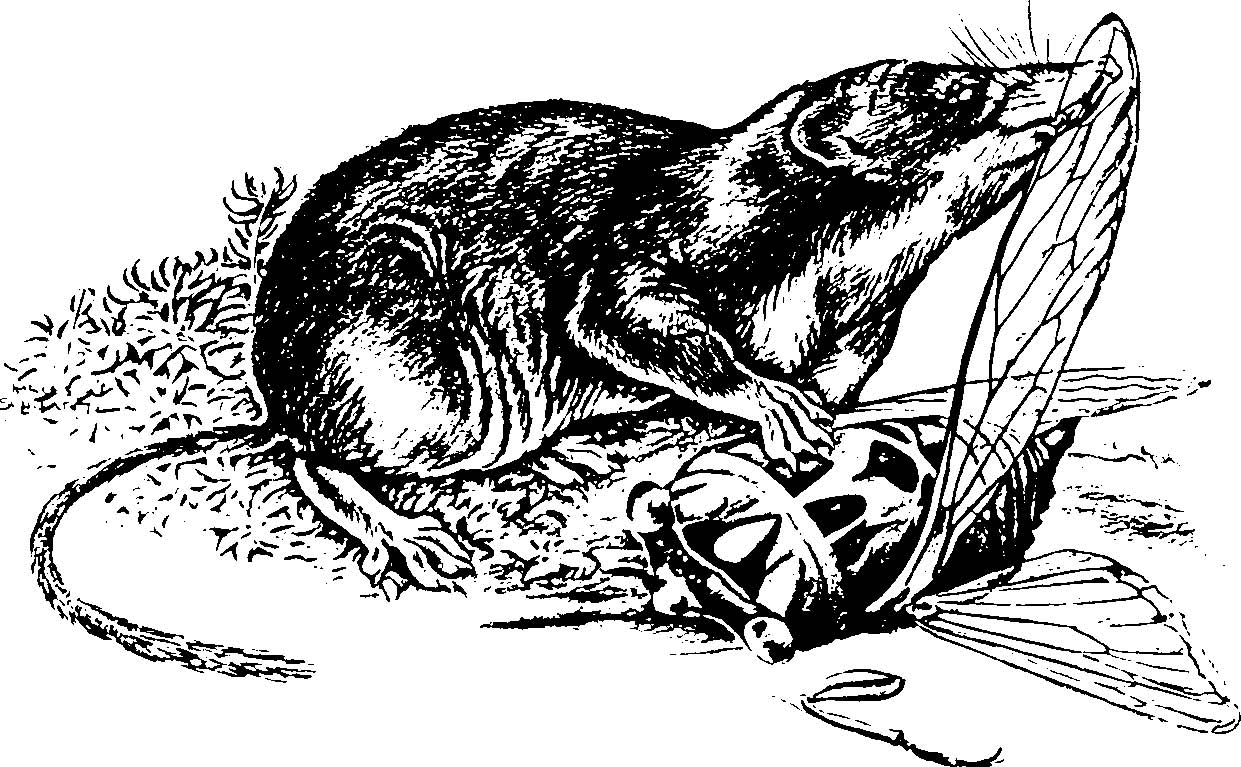
Fig. 1. A masked shrew,
Sorex cinereus
Identification
The shrew is a small,
mouse-sized mammal with an elongated snout, a dense fur
of uniform color, small eyes, and five clawed toes on
each foot (Fig. 1). Its skull, compared to that of
rodents, is long, narrow, and lacks the zygomatic arch
on the lateral side characteristic of rodents. The teeth
are small, sharp, and commonly dark-tipped. Pigmentation
on the tips of the teeth is caused by deposition of iron
in the outer enamel. This deposition may increase the
teeth’s resistance to wear, an obvious advantage for
permanent teeth that do not continue to grow in response
to wear. The house shrew (Suncus murinus) lacks the
pigmented teeth. Shrew feces are often corkscrew-shaped,
and some shrews (for example, the desert shrew [Notiosorex
crawfordi]) use regular defecation stations. Albino
shrews occur occasionally. Shrews are similar to mice
except that mice have four toes on their front feet,
larger eyes, bicolored fur, and lack an elongated snout.
Moles also are similar to shrews, but are usually larger
and have enlarged front feet. Both shrews and moles are
insectivores, whereas mice are rodents.
Worldwide, over 250
species of shrews are recognized, with over 30 species
recognized in the United States, the US Territories, and
Canada (Table 1). Specific identification of shrews may
be difficult. Taxonomists are still refining the
phylogenetic relationships between populations of
shrews. Consult a regional reference book on mammals, or
seek assistance from a qualified mammalogist.
Range
Shrews are broadly
distributed throughout the world and North America. For
specific range information, refer to one of the many
references available on mammal distribution for your
region. Publications by Burt and Grossenheider (1976),
Hall (1981), and Junge and Hoffmann (1981) are
particularly helpful.
Habitat
Shrews vary widely in
habitat preferences throughout North America. Shrews
exist in practically all terrestrial habitats, from
montane or boreal regions to arid areas. The northern
water shrew (Sorex palustris) prefers marshy or
semiaquatic areas. Regional reference books will help
identify specific habitats. A word of caution is in
order, however. Distribution studies based on the
results of snap-trapping research have a pronounced
tendency to understate the abundance of shrews. Studies
using pit traps are more successful in assessing the
presence or absence of shrews in a particular location.
Food Habits
Shrews are in the
taxonomic order Insectivora. As the name implies,
insects make up a large portion of the typical shrew
diet. Food habit studies have revealed that shrews eat
beetles, grasshoppers, butterfly and moth larvae,
ichneumonid wasps, crickets, spiders, snails,
earthworms, slugs, centipedes, and millipedes. Shrews
also eat small birds, mice, small snakes, and even other
shrews when the opportunity presents itself. Seeds,
roots, and other vegetable matter are also eaten by some
species of shrews.
General Biology, Reproduction, and Behavior
Shrews are among the
world’s smallest mammals. The pigmy shrew (Sorex hoyi)
is the smallest North American mammal. It can weigh as
little as 0.1 ounce (2 g). Because of their small size,
shrews have a proportionally high sur-face-to-volume
ratio and lose body heat rapidly. Thus, to maintain a
constant body temperature, they have a high metabolic
rate and need to consume food as often as every 3 to 4
hours. Some shrews will consume three times their body
weight in food over a 24-hour period.
Shrews usually do not live
longer than 1 to 2 years, but they have 1 to 3 litters
per year with 2 to 10 young per litter. Specific
demographic features vary with the species. The
gestation period is approximately 21 days.
Shrews have an acute sense
of touch, hearing, and smell, with vision playing a
relatively minor role. Some species of shrews use a
series of high-pitched squeaks for echolocation, much as
bats do. However, shrews probably use echolocation more
for investigating their habitat than for searching out
food. Glands located on the hindquarters of shrews have
a pungent odor and probably function as sexual
attractants. Blarina brevicauda, and presumably B.
carolinensis and B. hylophaga (the short-tailed shrews),
have a toxic venom in their saliva that may help them
subdue small prey.
Some shrews are mostly
nocturnal; others are active throughout the day and
night. They frequently use the tunnels made by voles and
moles. During periods of occasional abundance, shrews
may have a strong, although temporary, negative impact
on mouse or insect populations. Many predators kill
shrews, but few actually eat them. Owls in particular
consume large numbers of shrews.
Some shrews exhibit
territorial behavior. Depending on the species and the
habitat, shrews range in density from 2 to 70
individuals per acre (1 to 30/ hectare) in North
America.
Damage
Most species of shrews do
not have significant negative impacts and are not
abundant enough to be considered pests (Schmidt 1984).
Shrews sometimes conflict with humans, however. The
vagrant shrew (Sorex vagrans) has been reported to
consume the seeds of Douglas-fir (Pseudotsuga menziesii),
although the seeds constitute a minor part of the diet.
The masked shrew (Sorex cinereus) destroyed from 0.3% to
10.5% of white spruce (Picea glauca) seeds marked over a
6-year period (Radvanyi 1970). Lodgepole pine (Pinus
contorta) seeds are also eaten by the masked shrew.
Radvanyi (1966, 1971) has published pictures of shrew,
mouse (Peromyscus, Microtus, and Clethrionomys spp.),
and chipmunk (Eutamias spp.) damage to lodgepole pine
seeds, and describes shrew damage to white spruce seeds.
The northern water shrew (Sorex
palustris) may cause local damage by consuming eggs or
small fish at hatcheries. The least shrew (Cryptotis
parva), also known as the bee shrew, sometimes enters
hives and destroys the young brood (Jackson 1961). The
northern short-tailed shrew (Blarina brevicauda) has
been reported to damage ginseng (Panax spp.) roots.
Short-tailed and masked shrews reportedly can climb
trees where they can feed on eggs or young birds in a
nest or consume suet in bird feeders.
The pugnacious nature of
shrews sometimes makes them a nuisance when they live in
or near dwellings. Shrews occasionally fall into window
wells, attack pets, attack birds or chipmunks at
feeders, feed on stored foods, contaminate stored foods
with feces and urine, and bite humans when improperly
handled. Potential exists for the transmission of
diseases and parasites, but this is poorly documented.
The house shrew (Suncus
murinus) is an introduced species to Guam. It has been
reported as a host for the rat flea (Xenopsylla cheopis)
which can carry the plague bacillus (Yersinia pestis) (Churchfield
1990). Compared to rat (Rattus spp.) numbers, however,
house shrew numbers are usually low, and risk of plague
transmission is probably minimal. The house shrew is
accustomed to living around humans and houses, which
increases its damage potential. It is considered smelly
and noisy, making incessant, shrill, clattering sounds
as it goes along (Churchfield 1990:149). On occasion it
destroys stored grain products.
Table 1. Shrews of the
United States, the US Territories, and Canada (from
Legal Status Banks et al. 1987, and Jones et al. 1992).

Shrews are not protected
by federal Scientific name Common name laws, with one
exception. The south-eastern shrew (Sorex longirostris
fischeri) is protected in the Great Dismal Swamp in
Virginia and North Carolina by the Endangered Species
Act of 1973. Nowak and Paradiso (1983:131) list the
following additional species or populations of concern:
Sorex preblei, Sorex trigonirostri, and Sorex merriami
in Oregon; Sorex trigonirostri eionis in Florida along
the Homossassee River; and Sorex palustris punctulatus
in the southern Appalachians.
Some states may have
special regulations regarding the collection or killing
of nongame mammals. Consult your local wildlife agency
or Cooperative Extension office for up-to-date
information.
Damage Prevention and Control Methods
Exclusion
Rodent-proofing will also exclude shrews from
entering structures. Place hardware cloth of 1/4-inch
(0.6-cm) mesh over potential entrances to exclude
shrews. The pygmy shrew (Sorex hoyi) may require a
smaller mesh. Coarse steel wool placed in small openings
can also exclude shrews.
Cultural Methods
Regular mowing around structures should decrease
preferred habitat and food, and may increase predation.
Where shrews are eating tree seeds, plant seedlings
instead to eliminate damage.
Repellents No
repellents are registered for use against shrews.
Toxicants No
toxicants are registered to poison shrews.
Fumigants
No fumigants are registered for use against shrews.
It would be impractical to use fumigants because of the
porous nature of typical shrew burrows.
Trapping
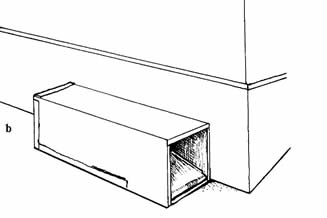
Mouse traps (snap traps), box traps, and pit traps
have been used to collect shrews. Set mouse traps in
runways or along walls, with the traps set at a right
angle to the runway and the triggers placed over the
runway (Fig. 2a). Small box traps can be set parallel to
and inside of runways, or parallel to walls around
structures (Fig. 2b). Bait the traps with a mixture of
peanut butter and rolled oats. A small amount of bacon
grease or hamburger may increase the attractiveness of
the bait.
A pit trap consists of a
gallon jar or a large can sunk into the ground under a
runway until the lip of the container is level with the
runway itself (Fig. 2c). Bait is not necessary. A small
amount of bacon grease smeared around the top of the
container may be an effective attractant, but this may
also attract large scavengers. Pit traps are more
effective for capturing shrews than snap traps, although
the increased labor involved in setting a pit trap may
not be justified when trying to capture only one or two
animals. Monitor pit traps daily, preferably in the
morning before the temperature gets hot, although
Churchfield (1990) recommends checking traps four times
in a 24-hour period. Place cotton wool in the pit trap
containers to reduce the mortality of trapped animals.
This is especially important to ensure the successful
release of nontarget animals. Since shrews are generally
beneficial in consuming insects, live-captured animals
can be relocated in suitable habitat more than 200 yards
(193 m) from the capture site.
The traps and placement
procedures described above are also effective for
catching mice. Note the identification characteristics
given above for determining whether the captured animal
is indeed a shrew. Sometimes birds are captured in traps
set for shrews. If this occurs, try placing a cover over
the traps, a cover over the bait, moving the traps to
another location, or omitting rolled oats from the bait
mixture.
Traps for Capturing Shrews
Fig. 2. Traps and trap
placement for capturing shrews: a) mouse trap (snap
trap) set perpendicular to wall, with trigger next to
wall; b) box trap set parallel to wall; c) pit trap sunk
in ground over runway (includes cotton wool).
A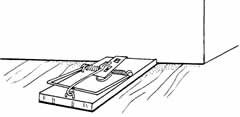
c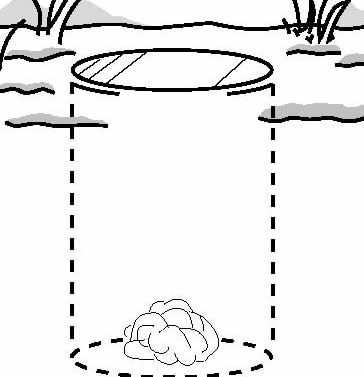
Shooting
Shooting is not practical and is not recommended. It
is illegal in some states and localities.
Other Methods
Owls may reduce local populations of shrews in poor
habitats, but this has not been documented. Domestic
cats appear to be very good predators of shrews,
although they seldom eat them (presumably because of the
shrew’s unpleasant odor). Cats may be effective at
temporarily reducing localized shrew populations living
in poor cover around structures. Cat owners may find
dead, uneaten shrews brought inside the home. Rather
than reduce the shrew population outside to prevent
this, simply monitor locations regularly used by your
cat, and dispose of dead shrews by placing a plastic bag
over your hand, picking up the dead animal, turning the
bag inside out while holding the shrew, sealing the bag,
and discarding it with the garbage. Using a plastic bag
in this manner reduces the potential for flea, tick,
helminth parasite, or disease transmission.
Economics of Damage and Control
No studies concerning the economics of shrew
damage and control are available. In Finland, shrews
appear to play a more important role as predators of
conifer seeds than they do in North America. Overall,
the economics of damage by shrews is not considered
great.
Folklore and Etymology
Chambers (1979) reviewed
some aspects of shrew biology and folklore: At one time
in Europe it was thought that if a shrew ran across a
farm animal that was lying down, the animal would suffer
intense pain. To counteract this, a shrew would be
walled up in an ash tree (a ‘shrew ash’), and then a
twig taken from the tree would be brushed onto the
suffering animal to relieve the pain. The ancient
Egyptians believed the shrew to be the spirit of
darkness. The shrew has also been mentioned as a Zuni
beast god, providing protection for stored grains from
raids by rats and mice (Hoffmeister 1967).
At least one tall tale
involving shrews has been found to be true. The
discovery that some shrews possess a toxic venom
confirms stories about the poisonous bite of shrews.
The etymology of the word
shrew is also interesting. The Old English form of the
word was screawa, or shrew-mouse. The Middle English
form was shrewe, meaning an evil or scolding person.
Thus shrew has a double meaning. It defines the small
mammal as well as an ill-tempered, scolding human
(usually female).
Shrews are in the family
Soricidae. Soricis is the genitive form of sorex, a
Latin word for shrew-mouse.
Acknowledgments
I appreciate the
assistance and comments of C. L. Shugart, S. E.
Hygnstrom, and four anonymous reviewers while developing
this manuscript. L. Thomas and J. Shepard provided
up-to-date information on the legal status of Sorex
longirostris fischeri.
Figure 1 is reproduced
with permission from Schwartz and Schwartz (1981).
Figure 2 was drawn by Jill
Sack Johnson.
For Additional Information
Banks, R. C., R. W. McDiarmid, and A. L. Gardner, eds.
1987. Checklist of vertebrates of the United States, the
US Territories, and Canada. US Dep. Inter, Fish Wildl.
Serv., Resour. Pub. 166. 79 pp.
Burt, W. H., and R. P.
Grossenheider. 1976. A field guide to the mammals.
Houghton Mifflin Co., Boston. 289 pp.
Chambers, K. A. 1979. A
country-lover’s guide to wildlife. The Johns Hopkins
Univ. Press, Baltimore, Maryland 228 pp.
Churchfield, S. 1990.
Thenatural history of shrews. Cornell Univ. Press,
Ithaca, New York. 178 pp.
Fowle, C. D., and R. Y.
Edwards. 1955. An unusual abundance of short-tailed
shrews, Blarina brevicauda. J. Mammal. 36:36-41.
Hall, E. R. 1981. The
mammals of North America. Vol. 1. John Wiley and Sons,
New York. 690 pp.
Hoffmeister, D. F. 1967.
An unusual concentration of shrews. J. Mammal.
48:462-464.
Jackson, H.H.T. 1961.
Mammals of Wisconsin. Univ. Wisconsin Press, Madison.
504 pp.
Jones, J. K., Jr., R. S.
Hoffmann, D. R. Rice, C. Jones, R. J. Baker, and M. D.
Engstrom. 1992. Revised checklist of North American
mammals north of Mexico, 1991. Occas. Papers Mus. Texas
Tech Univ. 146:1-23.
Junge, J. A., and R. S.
Hoffmann. 1981. An annotated key to the long-tailed
shrews (genus Sorex) of the United States and Canada,
with notes on Middle American Sorex. Univ. Kansas, Mus.
Nat. Hist., Occas. Papers 94:1-48.
Martin, I. G. 1981. Venom
of the short-tailed shrew (Blarina brevicauda) as an
insect immobilizing agent. J. Mammal. 62:189-192.
Nowak, R. M., and J. L.
Paradiso. 1983. Walker’s mammals of the world. Vol. 1.
The Johns Hopkins Univ. Press, Baltimore, Maryland. 568
pp.
Radvanyi, A. 1966.
Destruction of radio-tagged seeds of white spruce by
small mammals during summer months. For. Sci.
12:307-315.
Radvanyi, A. 1970. Small
mammals and regeneration of white spruce forests in
western Alberta. Ecol. 51:1102-1105.
Radvanyi, A. 1971.
Lodgepole pine seed depredation by small mammals in
western Alberta. For. Sci. 17:213-217.
Schmidt, R. H. 1984. Shrew
damage and control: a review. Proc. Eastern Wildl.
Damage Control Conf. 1:143-146.
Schwartz, C. W., and E. R.
Schwartz. 1981. The wild mammals of Missouri, rev. ed.
Univ. Missouri Press, Columbia. 356 pp.
Tomasi, T. E. 1979.
Echolocation by the short-tailed shrew Blarina
brevicauda. J. Mammal. 60:751-759.
Editors
Scott E. Hygnstrom Robert M. Timm Gary E.
Larson
PREVENTION AND CONTROL OF
WILDLIFE DAMAGE — 1994
Cooperative Extension
Division Institute of Agriculture and Natural Resources
University of Nebraska -Lincoln
United States Department
of Agriculture Animal and Plant Health Inspection
Service Animal Damage Control
Great Plains Agricultural
Council Wildlife Committee
Special
thanks to:
Clemson University
|