|
|
|
|
 |
DAMAGE IDENTIFICATION |
|
Wildlife Diseases and Humans |
INTRODUCTION
Diseases of wildlife can cause significant illness
and death to individual animals and can significantly
affect wildlife populations. Wildlife species can also
serve as natural hosts for certain diseases that affect
humans (zoonoses). The disease agents or parasites that
cause these zoonotic diseases can be contracted from
wildlife directly by bites or contamination, or
indirectly through the bite of arthropod vectors such as
mosquitoes, ticks, fleas, and mites that have previously
fed on an infected animal. These zoonotic diseases are
primarily diseases acquired within a specific locality,
and secondarily, diseases of occupation and avocation.
Biologists, field assistants, hunters, and other
individuals who work directly with wildlife have an
increased risk of acquiring these diseases directly from
animal hosts or their ectoparasites. Plague, tularemia,
and leptospirosis have been acquired in the handling and
skinning of rodents, rabbits, and carnivores. Humans
have usually acquired diseases like Colorado tick fever,
Rocky Mountain spotted fever, and Lyme disease because
they have spent time in optimal habitats of disease
vectors and hosts. Therefore, some general precautions
should be taken to reduce risks of exposure and prevent
infection.
GENERAL PRECAUTIONS
Use extreme
caution when approaching or handling a wild animal that
looks sick or abnormal to guard against those diseases
contracted directly from wildlife. Procedures for basic
personal hygiene and cleanliness of equipment are
important for any activity but become a matter of major
health concern when handling animals or their products
that could be infected with disease agents. Some of the
important precautions are:
-
Wear
protective clothing, particularly disposable rubber
or plastic gloves, when dissecting or skinning wild
animals.
-
Scrub
the work area, knives, other tools, and reusable
gloves with soap or detergent followed by
disinfection with diluted household bleach.
-
Avoid
eating and drinking while handling or skinning
animals and wash hands thoroughly when finished.
-
Safely
dispose of carcasses and tissues as well as any
contaminated disposable items like plastic gloves.
-
Cook
meat from wild game thoroughly before eating.
-
Contact a physician if you become sick following
exposure to a wild animal or its ectoparasites.
Inform the physician of your possible exposure to a
zoonotic disease.
Precautions against acquiring fungal diseases,
especially histoplasmosis, should be taken when working
in high-risk sites that contain contaminated soil or
accumulations of animal feces; for example, under large
bird roosts or in buildings or caves containing bat
colonies. Wear protective masks to reduce or prevent the
inhalation of fungal spores.
Protection from
vector-borne diseases in high-risk areas involves
personal measures such as using mosquito or tick
repellents, wearing special clothing, or simply tucking
pant cuffs into socks to increase the chance of finding
crawling ticks before they attach. Additional preventive
methods include checking your clothing and body and your
pets for ticks and removing the ticks promptly after
returning from infested sites. If possible, avoid
tick-in-fested areas or locations with intense mosquito
activity during the transmission season. Reduce outdoor
exposure to mosquitoes especially in early evening hours
to diminish the risk of infection with mosquito-borne
diseases.
Equally important
preventive measures are knowledge of the diseases
present in the general area and the specific habitats
and times of year that present the greatest risk of
exposure. Knowledge of and recognition of the early
symptoms of the diseases and the conditions of exposure
are essential in preventing severe illness.
Also important are medical
evaluation and treatment with proper antibiotics. For
example, if you become ill following some field activity
in a known plague-endemic area and you recognize the
early symptoms of the disease, seeking medical care and
informing the attending physician of your possible
exposure to plague will aid in the correct treatment of
your illness and reduce the risk of complications or
even death.
In addition to taking
personal precautions, risk of acquiring vector-borne
diseases can be reduced in specific locations through
area-wide applications of insecticides to control
mosquito or flea vectors or acaricides to control tick
vectors. Reduction in host populations (for example,
rodents) and their ectoparasites (fleas or ticks) may be
needed to control transmission of such diseases as
plague or Lyme disease. Vaccination of wildlife hosts as
a means of reducing zoonotic diseases is currently being
investigated and may soon be available for diseases like
rabies.
WILDLIFE DISEASES OF
PUBLIC HEALTH CONCERN
Directly Transmitted
Diseases
Rabies
Rabies is an acute
disease, caused by a virus (rhabdovirus), that can
infect all warm-blooded animals, and is usually fatal.
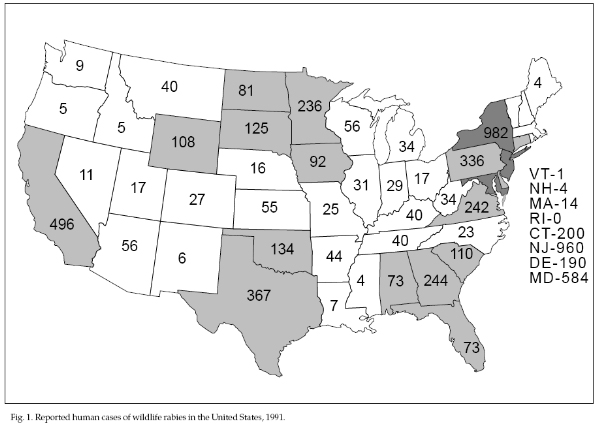
Certain carnivorous mammals and bats are the usual
animal hosts (Fig. 1; Table 1). Rabies occurs throughout
most of the world; only Australia and Antarctica are
free of it. Most human cases have been contracted from
rabies-infected dogs. In the United States, human cases
have decreased to an average of one person per year (75%
of cases are acquired outside the United States).
Reduction in human rabies is likely linked with the
intensive control of dog rabies during the 1950s and
1960s through massive vaccination campaigns, stray dog
control programs, and improvement in human treatment
following exposure. Nevertheless, thousands of people in
the United States continue to receive treatment every
year for possible exposure to rabies virus by animal
bites. Most of the treatments are still due to dog and
cat bites; however, these pet species have the lowest
occurrence of reported rabies among all animal species
tested.
Rabies in wildlife
increased dramatically during the 1960s and now accounts
for most of the reported animal rabies cases (91% in
1991). Some of the increase in reporting was due to real
increases in the number of cases, and some was due to an
increased awareness of wildlife rabies, particularly in
striped skunks, raccoons, and bats. In 1991, 6,975 cases
of animal rabies were reported in 49 states, the
District of Columbia, and Puerto Rico. Raccoons (44.2%),
striped skunks (29.7%), and various species of bats
(9.9%) continued to be the major hosts. Red and gray
foxes (4.6%), other wildlife species (2.8%), and
domestic animals (8.9%) comprise the remainder of hosts.
During the last 2 years, raccoons replaced striped
skunks as the major wildlife host in the United States
because of the continued expansion of raccoon rabies in
the northeastern United States. Animal cases are
reported throughout the year, although the number of
cases reported reaches a seasonal peak for skunks in
March and April, for raccoons in April, and for bats in
August.
Clinical Signs.
Rabies is considered almost 100% fatal once clinical
signs develop. The disease progresses rapidly following
the appearance of clinical signs, and the animal dies
within a few days. Although abnormal behavior is not
diagnostic for rabies (other diseases, like distemper,
cause similar behavioral changes), atypical behavior and
signs develop following brain infection, and rabies
should be suspected whenever wild animals display
unusual behavior.
Infected animals usually
display either “furious” or “dumb” rabies, although some
animals progress through both stages. Skunks, raccoons,
foxes, and other canids usually have furious rabies and
are unduly aggressive before convulsions and paralysis
set in. Some animals, however, have dumb rabies and
proceed to tremors and convulsions without agitation or
aggression. Other behavioral changes include
friendliness or loss of fear, appearance in the daytime
for some typically nocturnal species (skunks, bats),
unprovoked attacks on anything that moves (including
inanimate objects), bewilderment, and aimless wandering.
Unusual barking, crying, and frothing at the mouth are
additional signs, which are the result of paralysis of
the throat muscles. Occasionally, rabid bats are
encountered prostrate or fluttering on the ground,
unable to fly; they should be handled with care because
they can still bite and transmit rabies. Some rabid
bats, particularly solitary species like the hoary bat,
are aggressive and have been known to attack people. In
domestic animals, rabies should be suspected if there is
any change in normal habits, such as sudden change in
disposition, failure to eat or drink, running into
objects, or paralysis.
Transmission.
Rabies virus is transmitted primarily via the saliva
during the bite of a rabid animal. However, other
methods of transmission are possible. Accidental
exposure of wounds or cuts to the saliva or tissues of
infected animals can occur. The virus is also present in
various body organs of infected animals, especially the
brain and salivary glands, which poses a health hazard
to persons who are field dressing or performing
necropsies on these animals. In addition, aerosol
exposure has occurred, although rarely, in caves
containing very large populations of infected bats.
Transmission between animals also occurs by ingestion of
infected tissues and by transplacental passage to
offspring.
When the virus enters the
tissue of a susceptible animal or human, it multiplies
at the bite or inoculation site and travels slowly up
nerve fibers to the part of the brain that controls the
bitten area. The virus multiplies there and spreads to
other parts of the brain and eventually produces a
variety of signs in the infected animal or person. The
virus also spreads from the brain to other tissues,
particularly to the salivary glands, where it multiplies
and is released into the saliva. The virus is
perpetuated in nature when an infected animal with virus
in its saliva bites another animal.
The virus is rarely
present in the salivary glands without first occurring
in the brain and is present in the saliva for only a few
days before clinical signs appear. Exceptions occur in a
few species of bats and in a unique African virus strain
found in dogs. The length of the incubation period (from
the time the animal is bitten until clinical rabies
appears) is usually 2 to 3 weeks, but varies from 10
days to several months.
Handling of Suspect
Animals and Diagnosis. Use caution when approaching
a suspected rabid animal since many are still aggressive
and can bite even if paralyzed. If the animal is still
alive, it should be killed humanely without damaging the
head. To confirm whether an animal is infected with
rabies, the animal must be submitted to the local health
department or state diagnostic laboratory for testing.
Avoid exposure to any sick
or dead animals that are suspected to have rabies.
Handle any dead animal with gloves or with a plastic bag
that can be turned inside-out to cover and contain the
animal. Avoid direct skin contact with the animal. For
large animals such as skunks and raccoons, remove the
head cautiously and seal it in a plastic bag, avoiding
contact or aerosol exposure. Seal the whole animal or
head inside an additional plastic bag (double) and keep
it cool at all times. Do not freeze the specimen unless
a delay of several days is anticipated before it is
examined for rabies. Disinfect gloves or knives that
were in contact with the animal with a strong detergent
or bleach or dispose of them.
For transport to the
laboratory, place the double-wrapped specimen in a
leak-proof container with a coolant (not wet ice). Send
the container by bus or other prearranged
transportation. Include information about the specimen
(species, date, geographic data, behavior) and the
names, addresses, and telephone numbers of the person
submitting the specimen and of anyone exposed to the
animal.
To test for rabies, a
fluorescent antibody (FA) test is performed directly on
brain tissue to distinguish rabies virus from other
disease agents (like distemper virus) that could be
present in the animal’s brain. In some states, brain
material is inoculated into mice to demonstrate virus
for those specimens that resulted in human exposure.
If a person or pet is
exposed to an animal suspected of having rabies but that
has not been captured, record a description of the
suspect animal (species, behavior) and provide the
description to public health officials or the attending
physician to determine possible treatment.
Prevention and
Treatment. The best treatment for rabies is
prevention. Individuals at high risk of exposure to
rabies, such as wildlife biologists, game wardens,
animal control officers, animal handlers, and
veterinarians should be vaccinated before potential
exposure. Safe and highly effective vaccines are
available through a physician or the local health
department.
First aid should
immediately be provided to a person who has been bitten
by or had contact with a potentially rabid animal. Scrub
the exposed site, including bite wounds, with soap and
water or water alone and flush thoroughly. Then apply a
strong first aid solution (iodine) or cream. First aid
treatment is the most effective method of preventing
infection by the rabies virus but should not preclude
medical attention from a physician, hospital emergency
room, or the local health department. Contact your
physician or health department as soon as possible to
determine dosage of rabies vaccine and whether
antirabies serum is required. Inform the health care
professionals about the rabid animal and the
circumstances of the exposure (species of animal
involved and its behavior, if the attack or bite from
the animal was provoked, and what type of first aid was
administered).
Hantavirus
Hantavirus includes a
group of viruses that can cause a febrile illness in
humans which can be accompanied by kidney, blood, or
respiratory ailments and can sometimes be fatal. The
febrile illness includes fever, headache, muscle aches,
nausea, vomiting, and lower back pain. Field and
commensal rodents are the natural reservoirs for viruses
in this group and these viruses are found worldwide.
Infected rodents shed virus in their urine, feces,
and/or saliva and can remain chronically infected. The
contaminated excreta from infected rodents are thought
to be the source of virus for aerosol and direct (animal
bite) transmission to other rodents and humans.
The recent discovery of a
possible new hantavirus in the southwestern United
States and its apparent increased virulence, has
heightened the awareness of and concern for
rodent-associated diseases. It produces produces
respiratory distress and potential death in humans.
Human cases and deaths from this viral infection were
first reported in 1993 in the Four Corners area of
Arizona, Colorado, New Mexico, and Utah and, more
recently, throughout the United States. Preliminary
information has incriminated the deer mouse (Peromyscus
maniculatus) as the natural reservoir and source of
human infection in that region. Individuals trapping and
handling small rodents in this region should take
increased precautions to reduce their exposure to this
virus. They should at least wear surgical gloves and
masks when processing rodents (contact CDC Hotline for
more detailed and thorough safety information). Rodent
control with careful handling and disposal of carcasses
should be instituted at campsites or in cabins before
they are occupied. The premises should be sprayed with
detergents or diluted bleach before thorough cleaning.
Wet-mopping is recommended. Dry sweeping and vacuuming
may increase risk of producing airborne particles.
Rodent harborage should be removed from premises and
from the surrounding area. Exclude rodents where
possible.
Trichinosis
Trichinosis may result in
diahrrea, sudden edema of the upper eyelids,
photophobia, muscle soreness and pain, skin lesions,
thirst, sweating, chills, and weakness. Other
respiratory and neurological symptoms may appear if
treatment is delayed.
Trichinosis is contracted
by eating infected meat which contains the encysted
parasites. The parasites may remain infectious in meat
which is raw or poorly cooked.
Trichinosis is caused by a
nematode parasite which produces the disease in humans
and domestic and wild animals. Evidence indicates that
nearly all mammals are susceptible to infections with
this parasite, which encysts in the muscle of the host
and is then transmitted through consumption of infected
flesh. As would be expected, the disease is most common
in wild carnivores and scavengers.
As with other wildlife
diseases, trichinosis is difficult to control in nature.
However, certain steps can be taken to decrease the
problem. Carcasses of carnivores and other meat-eating
species should not be discarded in the fields or woods,
but should be made unavailable by burying or other
means. These carcasses also should not be fed to swine,
dogs, or other domestic animals. Open garbage dumps
should be replaced by the landfill type or other methods
of disposal where wildlife will not have access to meat
scraps. If open garbage dumps cannot be eliminated,
rodent control programs should be initiated and the
areas fenced to prevent scavenging by larger animals
such as foxes. These steps would markedly reduce the
problem of trichinosis in wildlife in the United States.
If carnivorous or
omnivorous wildlife such as bears, bobcats, opossums,
raccoons, or feral pigs are consumed by humans, the meat
should be properly prepared by cooking, freezing, or
curing to destroy any viable trichinae. Cooking to an
internal temperature of 137oF is deemed sufficient for
pork, while freezing at 5oF for 20 days, -10oF for 10
days, or 20oF for 6 days will kill trichinae. Curing
should follow approved government regulations.
Mosquito-borne
Encephalitis
Encephalitis is a disease
caused by mosquito-borne viruses (arboviruses) that
affect the central nervous system. Infections range from
unapparent to mild, nonspecific illnesses (fever,
headache, musculoskeletal pain, and malaise) to
occasionally severe illness of the central nervous
system resulting in permanent neurologic damage and
possibly death. The four major types of encephalitis in
the United States include St. Louis encephalitis (SLE),
California encephalitis (CE primarily includes the
LaCrosse virus [LAC]), eastern equine encephalitis (EEE),
and western equine encephalitis (WEE). The distribution
of these arboviruses varies (Fig. 2). SLE occurs
throughout the United States (an epidemic occurred in
central Florida in 1990 and Arkansas in 1991), WEE
occurs west of the Mississippi River, EEE occurs east of
the Mississippi River but mostly along the Atlantic and
Gulf coasts and north-central states, and CE occurs in
California and the eastern United States (LAC type).
Human cases of arbovirus infection have a seasonal
occurrence from mid- to late summer.
These distinct viruses
naturally infect a variety of birds and mammals and are
transmitted between animals by mosquito vectors.
Occasionally, infected mosquitoes will feed on human or
equine hosts that are “dead ends” for the viruses, with
little or no chance of subsequent transmission to other
mosquitoes. These viral infections may, however, result
in severe illness or death in humans or horses (EEE and
WEE). Only EEE and occasionally WEE viruses adversely
affect wild vertebrates; for example, EEE causes death
in ring-necked pheasants and other exotic game birds,
house sparrows, red-winged blackbirds, whooping cranes,
and other species. The wildlife hosts for LAC virus are
the eastern chipmunk, tree squirrels, and foxes. The
natural hosts for the other three viruses are mostly
songbirds, although squirrels and jackrabbits may be
involved in WEE transmission.
No treatment or commercial
vaccine is available for humans, but vaccines for WEE
and EEE are readily available for horses. The best
preventive measures are personal protection against
mosquito bites, especially avoiding exposure to
mosquitoes during early evening hours, and the use of
repellents. Mosquito populations can be reduced in an
area by eliminating breeding sites for vector species.
Killing adult mosquitoes with areawide applications of
insecticides has been most effective in preventing
epidemics.
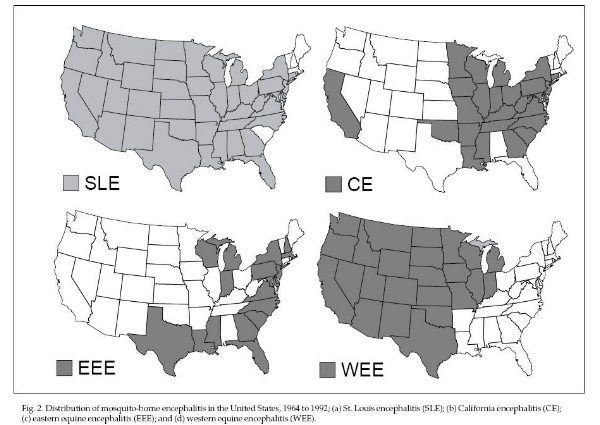
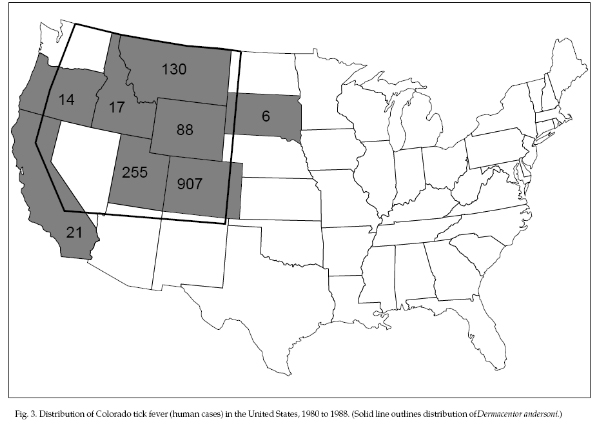
Tick-borne Diseases
Colorado
Tick Fever
Colorado tick fever (CTF)
is an acute and rather benign disease caused by a virus
(coltivirus) that is transmitted to humans by ticks.
Symptoms are usually limited to high fever, headache,
muscle aches, and lethargy, but the symptoms are
frequently biphasic and recurring. The disease is
confined to the mountains or highland regions of eight
western states and western Canada (Fig. 3). About 150 to
200 cases are reported each year; 1,438 cases were
reported from 1980 to 1988 in eight western states, 63%
of them in Colorado. CTF is transmitted to humans during
the spring and early summer by the bite of the adult
stage of the Rocky Mountain wood tick (Dermacentor
andersoni) or by D. occidentalis in California. The
virus is maintained in nature through transmission by
immature stages of ticks to various species of small
mammals, particularly chipmunks, ground squirrels, and
deer mice during the spring and summer months. The virus
survives the winter in infected tick nymphs and adults.
The habitats that support the rodent hosts and tick
vectors of the virus in the disease endemic region
contain rocky surfaces with moderate shrub cover and
scattered pines.
Avoid tick-infested
habitats during spring and early summer and use personal
protection against ticks. No vaccines or treatment are
available.
Rocky
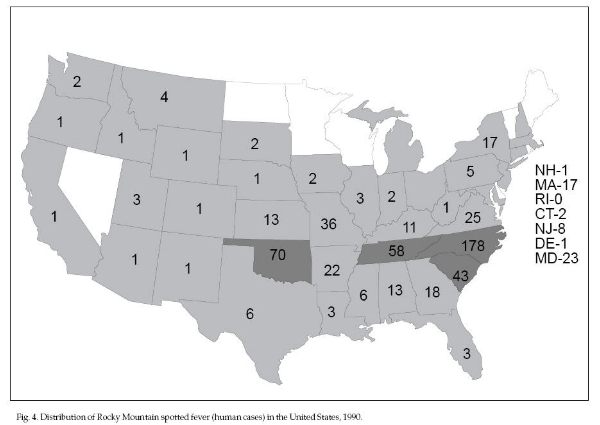
Mountain Spotted Fever (Tick-borne Typhus)
Rocky Mountain spotted
fever (RMSF) is a moderate to severe illness caused by a
rickettsia (Rickettsia rickettsii). The disease
is distinguished by a sudden onset of high fever, severe
headache, muscle pain, and a red rash starting on the
extremities about 3 to 6 days after onset of symptoms
and extending to the palms of hands and soles of feet
and then to the rest of the body. Delirium, coma, and
death occur in about 1% to 2% of cases (15% to 20% in
untreated cases). The disease is transmitted to humans
in the United States by several hard tick (Ixodidae)
species; D. andersoni in the Rocky Mountain region,
D. variabilis in the east and southeast, and
Amblyomma americanum in the south-central states. In
1990, 649 cases of RMSF were reported from all regions
of the United States, although more cases were reported
in the south-Atlantic and south-central states (Fig. 4).
The natural hosts for the rickettsia are a variety of
wild rodents, although rabbits and wild and domestic
carnivores are involved in some cases. The rickettsia
survive the winter months in the tick vector and may be
maintained by transovarial transmission from the female
adult tick to its offspring.
Avoid tick-infested areas
and use personal measures to protect against tick bites.
No vaccine is presently licensed for public use, but
antibiotic treatment is effective and should be
initiated without waiting for laboratory confirmation of
clinical diagnosis.
Lyme
Disease
Lyme disease is caused by
a spirochete bacterium (Borrelia burgdorferi)
that is transmitted to humans by hard ticks. Early
symptoms include a flu-like illness with headache,
slight fever, muscle or joint pain, neck stiffness,
swollen glands, jaw discomfort, and inflammation of the
eye membranes. A diagnostic rash, erythema migrans (EM),
occurs in 65% to 75% of the cases. The rapidly expanding
red rash starts at the tick bite site and expands to a
nearly circular lesion of about 1 to 8 inches (2 to 20
cm). It often has a bulls-eye appearance with central
clearing and/or darkening around the edge. Additional
smaller skin lesions may appear at other sites of the
body and may last for days or weeks. Later symptoms,
including heart, nervous system, and joint
manifestations, may develop in untreated individuals.
The joint pain and swelling usually occur one or more
months after infection, may involve one or more joints,
and may recur in different joints; the knee joint is
most frequently affected. Domestic animals may be
affected as well.
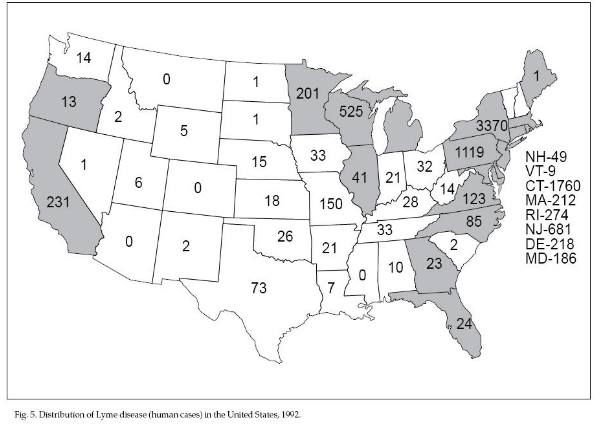
In 1992, 9,695 cases of
Lyme disease were reported in 44 states (Fig. 5). Most
cases were reported in the northeastern and upper
midwestern states where the vector is the deer tick (Ixodes
scapularis) and where transmission is predominately in
residential communities. Other vectors are I. pacificus
on the West Coast and possibly A. americanum in the
Southeast and in south-central states. Transmission in
these other regions of the United States may be more
sporadic and occur during outdoor activities related to
recreation and occupation. Acquisition of Lyme disease
by humans peaks during the summer months when the tick
nymphs are feeding on hosts. Because of its small size,
the attached nymph frequently goes unnoticed and is not
removed. The transmission cycle of Lyme disease begins
when larvae acquire spirochetes while feeding on
infected white-footed mice, chipmunks, other rodents,
and birds. Engorged larvae drop to the ground, molt to
the nymphal stage, and wait until the following summer
to attach to and transmit spirochetes to susceptible
rodents, birds, larger mammals, and humans. Uninfected
larvae subsequently feed on these wild vertebrate hosts
to complete the transmission cycle. The engorged nymphs
drop to the ground and molt into adult ticks which are
active during the fall and following spring and feed on
large mammals, primarily deer. Deciduous forest is the
predominant habitat for the tick vector and vertebrate
hosts in the Northeast and Midwest. Other prime habitats
include forested areas interspersed with residential
development and grass and shrub areas, particularly
along forest edges.
Patients treated with
appropriate antibiotics during the early stages of the
disease usually have rapid and complete recovery. Even
patients treated during later stages generally respond
well and recover. No vaccine is available except for
domestic dogs. Avoid locations with ticks during
seasonal activity periods, use personal measures to
protect against ticks, become knowledgeable about the
symptoms of Lyme disease, and seek medical care and
treatment if infected.
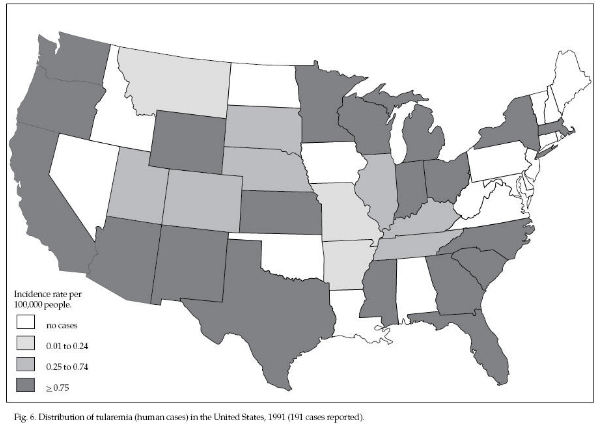
Tularemia
Tularemia is caused by the
bacteria Francisella tularensis and is characterized by
sudden onset of high fever and chills, joint and muscle
pain, and prostration. Slow-healing sores or lesions
develop at the site of entry of the bacteria (or
arthropod bite). Inflammation and swelling of nearby
lymph nodes follow.
Tularemia is endemic
throughout North America (Fig. 6). Most of the 100 to
300 cases reported each year are from the area between
the Rocky Mountains and the Mississippi River
(especially Arkansas and Missouri). Most cases are
acquired during the summer months from vector
transmission; however, a second peak of cases occurs
during the winter and is probably associated with rabbit
hunting and carnivore trapping.
The bacteria is maintained
in rabbits, hares, rodents, and birds by tick
transmission. The natural reservoir for the bacteria
includes infected ticks and animal species that are less
susceptible and thus survive acute infections. Hard
ticks, primarily D. andersoni, D. variabilis, and
Haemaphysalis leporispalustris, and some flies,
especially the deerfly (Chrysops discalis), can
subsequently transmit the disease to humans. Tularemia
can also be transmitted directly to humans. Transmission
routes include drinking contaminated water; eating
contaminated food or improperly cooked game meat;
inhaling aerosols contaminated with rodent urine, feces,
or dust; cuts from contaminated knives or other
instruments; and scratches or bites from infected
animals. Use personal protection measures against ticks
and practice good sanitation procedures when handling
wild animals, especially rabbits. Promptly seek medical
care and treatment if symptoms develop.
Relapsing
Fever
Relapsing fever can be
caused by several Borrelia spirochete bacteria, which
are related to the Lyme disease spirochete and are
transmitted by soft ticks (Argasidae). Symptoms resemble
Lyme disease except for the absence of the diagnostic
rash and the presence of recurring fever. The most
common type is caused by B. hermsii. Most human cases of
this type of relapsing fever have been associated with
log cabins or houses containing rodent nests
(particularly of chipmunks and pine squirrels) and
Ornithodoros hermsi ticks. This species of tick is
active at night. Since it feeds rapidly and its bite is
relatively painless, it may go unnoticed. The ticks feed
on humans when the rodents disappear from the cabin
nests because of rodent control measures or death from
other diseases. Most human cases occur during the summer
months when the cabins are in use. Sporadic cases are
reported primarily in the mountainous regions of the
western United States and British Columbia; 159 cases
were reported during 1985 to 1991 in 10 western states
(Fig. 7). Two outbreaks occurred among tourists and
staff staying in cabins at the Grand Canyon in Arizona
in 1973 and 1990. Inspect cabins for rodent use and
nests, promptly remove nests, and treat cabins with
insecticides or fumigate to kill any remaining ticks.
Rodent-proof cabins to prevent rodent entry.
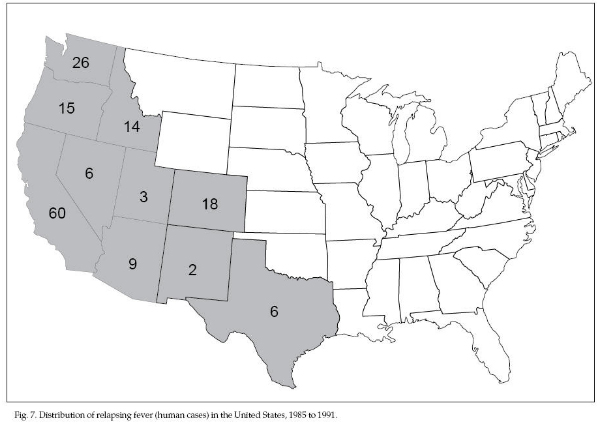
Two other species of
relapsing fever spirochetes are transmitted occasionally
to humans in the western United States by Ornithodoros
ticks. The spirochete B. parkeri is transmitted by O.
parkeri, mostly in California, and B. turicatae by the
tick O. turicata. Five humans were infected with B.
turicatae in Texas in 1990 following exploration of a
cave containing infected ticks. For prevention, use
personal protection against tick exposure. If sick with
relapsing fever, seek medical care and appropriate
antibiotic treatment.
Other
Tick-borne Diseases
Three other tick-borne
diseases occur in the United States. Human ehrlichiosis
is a recently recognized disease caused by a rickettsia,
Ehrlichia chaffeensis. It is probably transmitted by
ticks. Symptoms are similar to those of RMSF: an acute
fever with headache, muscle ache, and nausea. A rash
appears less frequently and for a much shorter duration.
From 1986 to 1991, 262 cases and 4 fatalities were
reported in 23 states, the majority occurring in
Missouri and Oklahoma. Use personal protection against
ticks and seek medical care and treatment if sick.
Powassan encephalitis is
caused by a virus (flavivirus) which is transmitted by
the ticks I. cookei, D. andersoni, and other Ixodes spp.
Symptoms include the sudden onset of fever, sore throat,
sleepiness, headache, and disorientation. Encephalitis,
meningitis, and, occasionally, partial paralysis may
develop. Natural hosts are marmots, sciurid rodents,
rabbits, hares, carnivores, and possibly birds. Only 19
cases have been reported, all in New York, Pennsylvania,
Ontario, and Quebec. Use personal protection to reduce
exposure to ticks. No treatment is available.
Babesiosis is a protozoan
disease with gradual onset of fever, sweating, loss of
appetite, fatigue, general muscle ache, and possibly
prolonged anemia. The disease can be severe and
sometimes fatal. A protozoan, Babesia microti, is
transmitted among wild rodents, particularly
white-footed mice, by the tick I. scapularis along the
coastal areas of New England and on adjacent offshore
islands. This tick may be infected occasionally with
both B. microti and the Lyme disease spirochete. Use
personal protection measures to prevent tick exposure
and seek medical care if sick.
Personal
Protection
The following personal
measures can protect against tick-transmitted diseases:
-
When possible, avoid
tick-infested areas.
-
To better see crawling
ticks, tuck pant legs into socks and tape the tops
of socks over pant legs. Wear light-colored clothes.
-
Use tick repellent on
exposed skin (DEET) or treat clothes with permethrin.
Follow label instructions for use.
-
Check yourself
frequently for ticks and remove them.
-
After outdoor
activity, remove and wash field clothing promptly
and dry clothes at a high temperature.
-
Inspect your body
carefully and remove attached ticks with a pointed
tweezers. Grasp ticks as close to the skin as
possible and pull them loose with a slow, steady
motion.
-
Inspect pets carefully
for ticks and remove ticks soon after returning from
the outdoors.
Flea-borne Diseases
Plague
Plague is an acute disease
caused by the bacteria Yersinia pestis. Humans usually
become infected by the bites of infected fleas but also
directly from exposure to tissues or body fluids from
diseased animals, especially when skinning animals. The
disease is characterized by the sudden onset of fever
and chills, followed by the development of swollen and
painful lymph nodes (buboes) in the armpits, groin, and
other areas 2 to 6 days following exposure. In addition
to the bubonic form, septicemic infection may develop
and involve other organs. Secondary infection of the
lungs may lead to primary plague pneumonia, which then
can be transmitted from person to person by aerosol. The
disease may be only mild and short-lived but frequently
progresses to a severe form, with 25% to 60% fatality in
untreated cases. In the United States, plague is
maintained in wild rodent populations in the western
states by flea transmission between rodents. Sylvatic
plague may persist in these animal populations with
varying severity, depending on the species’ resistance.
Prairie dogs are susceptible to sudden die-offs.
Outbreaks of plague have decimated prairie dog colonies
in less than 1 to 2 years. Rabbits, hares, carnivores,
and wild ungulates have also been infected occasionally.
Human cases of plague are reported most frequently in
New Mexico, Arizona, California, Colorado, and Oregon
(Fig. 8). More than 50% of the 284 cases in the United
States reported from 1970 to 1990 were in New Mexico.
Use insect repellents on skin or treat field clothes
with permethrin. Practice good sanitation procedures
when handling animals. Seek medical care and treatment
if sick.
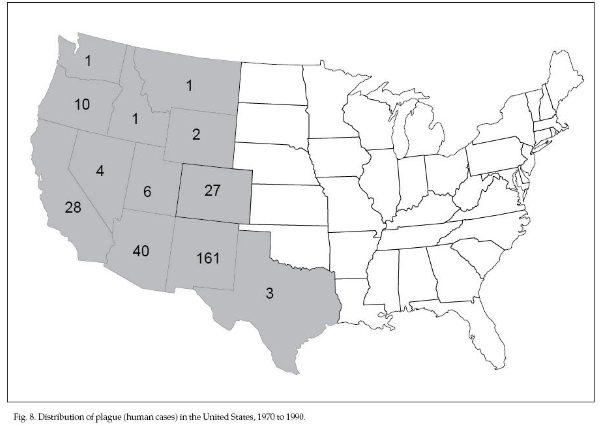
Murine
Typhus Fever
Murine typhus fever is
caused by Rickettsia typhi, a rickettsial
organism that occurs throughout the southeastern and
Gulf Coast states and southern California. Rats are the
reservoir animals from which the disease reaches many
humans by way of rat fleas. The oriental rat flea,
Xenopsylla cheopis, is considered the most important
vector of the disease. The causative organism enters the
bloodstream when feces of infected fleas are scratched
or rubbed into a flea-bite wound or other breaks in the
skin. Murine typhus is similar to epidemic or
louse-borne typhus, but illness is much milder and the
fatality rate in untreated cases is much lower.
Commensal Rodent-borne
Diseases
Rats and mice are
responsible for the spread of over 35 diseases, either
directly, through contamination of human food with their
urine or feces, or indirectly, by way of rodent fleas
and mites. Following are brief descriptions of the more
common of these diseases.
Rat-bite
Fever
Rat-bite fever is caused
by the bacteria Streptobacillus moniliformis, which is
found on the teeth and gums of rats. It is transferred
from rats to humans by the bite of the rat. The most
frequently occurring rat-bite fever in the United States
is called Haverhill fever. It is similar to the rat-bite
fever of the Orient called sodoku (caused by Spirillus
minus).
Leptospirosis (Weil’s Disease)
Leptospirosis is a mild to
severe infection that is seldom fatal. Human cases of
the disease result from direct or indirect contact with
infected urine of rodents and other animals. The
spirochetes (Leptospira spp., primarily L.
icterohemorrhagiae) are found in contaminated water or
on food, and may enter humans through mucous membranes
or minute cuts or abrasions of the skin. Thus, Weil’s
disease is often found in sailors, miners, sewer
workers, and fish or poultry dealers. In a recent study
in Hawaii, Norway rats, roof rats, and house mice were
found to have high L. icterohemorrhagiae carrier rates.
Symptoms of leptospirosis
infection range from none to severe, with acute
fatalities. Many infections are characterized by
diarrhea, chills, vomiting, myalgia, and kidney damage.
Prevention is the most important means of dealing with
this disease. Proper sanitation, rodent-proofing, and
food storage and handling are essential. Medical
attention is typically required.
Salmonellosis
The Salmonella group of
bacteria exists nearly everywhere in the environment
and, unfortunately, several serotypes are pathogenic to
humans and other animals. Salmonellosis can lead to
severe cases of gastroenteritis (food poisoning),
enteric fever septicemia (blood poisoning), and death.
Food poisoning, the most common malady, is characterized
by a sudden onset of abdominal pain, diahrrea, nausea,
and vomiting. Due to the severity of this disease,
medical attention is typically required.
Salmonella bacteria
recognize few host barriers and are transmitted in many
ways. One common form of transmission is through food
contaminated by rat or mouse feces that contain
Salmonella (especially S. typhimurium) organisms. It may
also be spread by birds, which contaminate food with
their feces or bacteria carried on their feet.
As with leptospirosis, the
most important means of reducing the potential of this
disease is through proper sanitation, rodent-proofing,
and food storage and handling. Rodent control through
trapping and appropriate use of toxicants may also be
necessary.
Rickettsialpox
Rickettsialpox is a mild
nonfatal disease resembling chicken pox. It is caused by
a rickettsia (Rickettsia akari), which is transmitted
from house mice to humans by the bite of an infected
house mouse mite (Liponyssoides sanguineus). In this
country rickettsialpox has been reported in Boston, West
Hartford, New York, Cleveland, and Philadelphia.
Bird-borne Diseases
Large roosting
concentrations of birds can be noisy, and the associated
droppings can be a nuisance because of the objectionable
odor and mess. In addition, birds may carry and transmit
diseases to livestock and humans. Collections of
droppings may provide a medium for bacterial and fungal
growth that could pose a potential public health
problem. Birds should be dispersed or controlled when
they form large concentrations near human habitations
and are judged to pose a threat to public health or
livestock. Concentrations of birds that do not threaten
human health or agriculture are usually better left
undisturbed.
Histoplasmosis
Histoplasmosis is a
respiratory disease in humans caused by inhaling spores
from the fungus Histoplasma capsula-tum. Birds do not
spread the disease directly — spores are spread by the
wind and the disease is contracted by inhalation. Bird
droppings enrich the soil and promote growth of the
fungus. Notable sources for histoplasmosis infection
include: (1) traditional bird roosts, (2) poultry farms,
(3) enclosed buildings where birds or bats have roosted,
and (4) natural or organic fertilizers. In addition, the
fungus can grow in various natural soils, with or
without droppings. In some areas, such as the Ohio
Valley, histoplasmosis is so widespread that 95% of the
human population becomes infected, whether associated
with birds or not.
Infection by only a few
spores generally produces a mild case in humans and
people are often unaware that they have contracted the
disease (unless it is detected later through a skin
reactivity test or lung X ray that reveals healed
lesions). A more severe infection may result in an acute
respiratory illness with flu-like symptoms (in fact,
histoplasmosis is often misdiagnosed as flu). The most
serious infections, usually resulting from massive spore
inhalation, may involve a dissemination of the fungus
through the blood stream. Such cases may become chronic,
recurring at later times, and affect organs other than
the lungs. Treatment with an antifungal agent such as
amphotericin B or imidazole ketoconazole may be
prescribed in more severe cases.
Not all blackbird or
starling roosts pose immediate public health problems
related to histoplasmosis. The histoplasmosis fungus
grows readily in the soil beneath bird roosts, but it
cannot form spores under the acidic conditions of fresh
droppings. An active, undisturbed roost may only give
off a few spores. Old or abandoned roosts, however, can
pose a significant threat to human health. After the
droppings have dried out or been leached by the rain,
the right conditions develop for spore release. If the
soil is stirred up under dusty conditions, as may be the
case in land clearing or bulldozing, massive amounts of
spores may be released. Severe epidemics have occurred
in association with bird roosts under such conditions.
Birds in large roosts can
be dispersed by the use of various frightening devices
or by roost thinning or clearing (see Bird Dispersal
Techniques). Precautions should be taken when working
around an old or abandoned roost site. It is wise to
test for the presence of histoplasmosis before beginning
any work. Wear a self-contained breathing apparatus or
face mask with a dust filter (less than 2 microns) to
prevent inhalation of the spores. Wear protective
clothing, gloves, and boots that can be removed and
disinfected with formalin and washed. If an area that
was once a bird roost is going to be cleared or
bulldozed, the area should be dampened with water or
work should be done when the weather is wet or cold or
both. Avoid working under dry, dusty conditions in late
summer. A roost may be decontaminated by spraying it
with a 3% to 5% solution of formaldehyde before
clearing, but this option is very expensive.
Ornithosis
(Chlamydia psittaci, psittacosis)
Ornithosis is an
infectious respiratory disease caused by Chlamydia
psittaci, a viruslike organism that affects humans,
pets, and livestock. It usually leads to a mild
pneumonia-or flu-like infection, but it can be a rapidly
fatal disease (less than 1% of the cases reported in the
United States). In humans many cases occur that are
undetected or incorrectly diagnosed. Pigeons are most
commonly associated with the transmission of ornithosis
to humans. Birds have adapted to the disease and show no
symptoms, but act as healthy carriers, shedding the
organism in their feces, which later may become airborne
as dust. The disease may also be contracted from
parakeets, farm poultry, or waterfowl.
People working in dry,
dusty areas where bird droppings are present, should
wear face masks or respirators to avoid inhaling
airborne avian fecal material. Spray work areas with
water and/or disinfectants to minimize the potential for
airborne infections particles. Medical attention,
including antibiotic treatments are recommended for
disease treatment.
Salmonellosis
The Salmonella group of
bacteria can also be transmitted by birds. Refer to
Commensal Rodent-borne Diseases (above) for additional
information.
Other
Bird-borne Diseases
Pigeons, starlings,
sparrows, blackbirds, and other types of birds have been
implicated in the transmission of various diseases of
significance to humans or livestock. Starlings have been
shown to be vectors of transmissible gastroenteritis (TGE)
of swine. The virus can be carried in an infective state
in the birds’ intestines or on their feet for up to 30
hours. It is generally fatal to baby pigs and causes
weight loss in adults. Starlings may also be involved in
the transmission of hog cholera. Cryptococcosis is a
fungal disease spread by pigeons and starlings that
results in chronic, usually fatal, meningitis. Various
species of birds may also play a part in the
transmission of encephalitis, Newcastle disease,
aspergillosis, toxoplasmosis, pseudotuberculosis, avian
tuberculosis, and coccidiosis.
Conclusion
Wildlife workers tend to
ignore the risks associated with handling wildlife
species and working in natural environments. Diseases of
wildlife or diseases present in their habitats can
infect humans and some can cause serious illness or even
death. Becoming aware of the potential diseases present
and taking precautions to decrease exposure will greatly
reduce chances of becoming infected with one of these
diseases. This section provides a description of the
major zoonotic diseases of wildlife in the United States
that can also infect humans and gives information on
disease prevention. Other diseases are briefly listed in
Table 1 or can be found in one of the selected
references.
You can prevent infection
with zoonotic diseases and reduce the seriousness of an
illness by observing the following recommendations:
-
Become aware of which
zoonotic diseases are present in your area and their
clinical symptoms.
-
Obtain any preexposure
vaccinations that are available, particularly for
rabies.
-
Take personal
precautions to reduce exposure to disease agents and
vectors such as ticks, mosquitoes, and fleas.
-
Practice good
sanitation procedures when handling or processing
animals or their products.
-
If you become ill,
promptly seek proper medical treatment and inform
the physician about possible exposures.
Special
thanks to:
Clemson University
Robert G. McLean
Chief, Vertebrate Ecology Section
Medical Entomology & Ecology Branch, Division of
Vector-borne Infectious, Diseases National Center for
Infectious Diseases, Centers for Disease Control and
Prevention, Fort Collins, Colorado 80522
Acknowledgments
Portions of this chapter were derived from F. R.
Henderson. 1983. Wildlife diseases and man. in R. M.
Timm, Prevention and Control of Wildlife Damage. Univ.
Nebraska Coop. Ext. Lincoln.
For
Additional Information
For further information, consult the local or state
health department or contact the CDC Voice Information
System, Centers for Disease Control and Prevention,
Atlanta, Georgia, at (404) 332-4555.
Acha, P. N., and B.
Szyfres. 1987. Zoonoses and communicable diseases common
to man and animals, 2d ed. Pan Am. Health
Org. Washington, DC. 963 pp.
Adrian, W. J., ed. 1981. Manual of common wildlife diseases in Colorado.
Colorado Div. Wildl. Denver. 139 pp.
Benenson, A. S., ed.
1990. Control of communicable diseases in man, 15th ed.
Am. Public Health Assoc. Washington, DC. 532
pp.
Thorne, E. T., N. Kingston, W. R. Jolley, and R. C. Bergstrom eds. 1982.
Diseases of wildlife in Wyoming, 2d ed. Wyoming Game
Fish Dep. Cheyenne. 353 pp.
Weeks, R. J., and A. R. Stickley, Jr. 1984. Histoplasmosis and its
relation to bird roosts: a review. Denver Wildl. Res.
Center.
Bird Damage Res. Rep. No. 330. Denver, Colorado. 23 pp.
Editors
Scott E. Hygnstrom, Robert M. Timm, Gary E. Larson
|