|
|
|
|
 |
BIRDS: European Starlings |
|
|

Fig. 1. European starling,
Sturnus vulgaris
Identification
Starlings are robin-sized
birds weighing about 3.2 ounces (90 g). Adults are dark
with light speckles on the feathers. The speckles may
not show at a distance (Fig. 1). The bill of both sexes
is yellow during the reproductive cycle (January to
June) and dark at other times. Juveniles are pale brown
to gray.
Starlings generally are
chunky and hump-backed in appearance, with a shape
similar to that of a meadowlark. The tail is short, and
the wings have a triangular shape when outstretched in
flight. Starling flight is direct and swift, not rising
and falling like the flight of many blackbirds.
Range
Since their introduction
into New York in the 1890s, starlings have spread across
the continental United States, northward to Alaska and
the southern half of Canada, and southward into northern
Mexico. They are native to Eurasia, but have also been
introduced in South Africa, Australia, New Zealand, and
elsewhere.
Habitat
Starlings are found in a
wide variety of habitats including cities, towns, farms,
ranches, open woodlands, fields, and lawns. Ideal
nesting habitat would include areas with trees or other
structures that have cavities suitable for nesting and
short grass (turf) areas or grazed pastures for
foraging. Ideal winter habitat would include areas with
structures and/or tall trees for daytime loafing
(resting) and nighttime roosting; and grazed pastures,
open water areas, and livestock facilities for foraging.
Food Habits
Starlings consume a
variety of foods, including fruits and seeds of both
wild and cultivated varieties. Insects, especially
Coleoptera and Lepidoptera lawn grubs, and other
invertebrates total about one-half of the diet overall,
and are especially important during the spring breeding
season. Other items including livestock rations and food
in garbage become an important food base for wintering
starlings.
General Biology, Reproduction, and Behavior
European starlings were
brought into the United States from Europe. They were
released in New York City in 1890 and 1891 by an
individual who wanted to introduce to the United States
all of the birds mentioned in Shakespeare’s works. Since
that time, they have increased in numbers and spread
across the country. They were first observed in Nebraska
in 1930, in Colorado in 1939, and in California in 1942.
The starling population in the United States is
estimated at 140 million birds.
Starlings nest in holes or
cavities almost anywhere, including tree cavities,
birdhouses, and holes in buildings or cliff faces.
Females lay 4 to 7 eggs which hatch after 11 to 13 days
of incubation. Young leave the nest when they are about
21 days old. Both parents help build the nest, incubate
the eggs, and feed the young. Sometimes 2 clutches of
eggs are laid per season, but most of the production is
from the first brood fledged.
Although starlings are not
always migratory, some will migrate up to several
hundred miles, while others may remain in the same
general area throughout the year. Hatching-year
starlings are more likely to migrate than adults, and
they tend to migrate farther.
Outside the breeding
season, starlings feed and roost together in flocks.
Starling and blackbird flocks often roost together in
urban landscape trees or in small dense woodlots or
overcrowded tree groves. They choose trees or groves
that offer ample perches so that all may roost together.
In colder weather they choose dense vegetation such as
coniferous trees or structures (such as barns, urban
structures) that provide protection from wind and cold.
Fall-roosting flocks are relatively small (from several
hundred to several thousand birds), but because they are
spread over large geographic areas, they can cause
widespread nuisance problems. In contrast,
winter-roosting flocks are large (sometimes exceeding 1
million birds), but are often confined to a few acres
(ha). Some of the winter roosting areas are occupied by
starlings year after year (Fig. 2). Each day they may
fly 15 to 30 or more miles (24 to 48 km) from roosting
to feeding sites. During the day when not feeding, they
may perch in smaller groups inside farm buildings or in
other warm, protected spots in and around urban
structures.
Damage and Damage Identification
Starlings are frequently
considered pests because of the problems they cause,
especially at livestock facilities (Fig. 3) and near
urban roosts. Starlings may selectively eat the
high-protein supplements that are often added to
livestock rations.
Starlings may also be
responsible for transferring disease from one livestock
facility to another. This is of particular concern to
swine producers. Tests have shown that the transmissible
gastroenteritis virus (TGE) can pass through the
digestive tract of a starling and be infectious in the
starling feces. Researchers, however, have also found
healthy swine in lots with infected starlings. This
indicates that even infected starlings may not always
transmit the disease, especially if starling interaction
with pigs is minimized. TGE may also be transmitted on
boots or vehicles, by stray animals, or by infected
swine added to the herd. Although starlings may be
involved in the spread of other livestock diseases,
their role in transmission of these diseases is not yet
understood.
Starlings cause other
damage by consuming cultivated fruits such as grapes,
peaches, blueberries, strawberries, figs, apples, and
cherries. They were recently found to damage ripening
(milk stage) corn, a problem primarily associated with
blackbirds. In
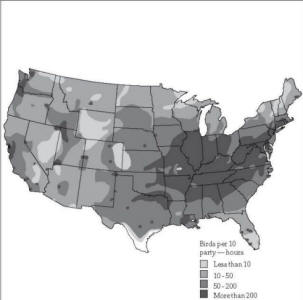
Fig. 2. Starling wintering
areas, 1972. Map by J. W. Rosahn, based on the National
Audubon Society’s annual Christmas Bird Count.

Fig. 3. At livestock facilities such as this pig
operation, European starlings consume feed, contaminate
feed and water with their droppings, and may transmit
disease.
some areas starlings pull
sprouting probe for grubs, but the frequency and grains,
particularly winter wheat, and extent of such damage is
not well eat the planted seed. Starlings may documented.
damage turf on golf courses as they The growing
urbanization of wintering starling flocks seeking warmth
and shelter for roosting may have serious consequences.
Large roosts that occur in buildings, industrial
structures, or, along with blackbird species, in trees
near homes are a problem in both rural and urban sites
because of health concerns, filth, noise, and odor. In
addition, slippery accumulations of droppings pose
safety hazards at industrial structures, and the acidity
of droppings is corrosive.
Starling and blackbird
roosts located near airports pose an aircraft safety
hazard because of the potential for birds to be ingested
into jet engines, resulting in aircraft damage or loss
and, at times, in human injuries. In 1960, an Electra
aircraft in Boston collided with a flock of starlings
soon after takeoff, resulting in a crash landing and 62
fatalities. Although only about 6% of bird-aircraft
strikes are associated with starlings or blackbirds,
these species represent a substantial management
challenge at airports.
One of the more serious
health concerns is the fungal respiratory disease
histoplasmosis. The fungus Histoplasma capsulatum may
grow in the soils beneath bird roosts, and spores become
airborne in dry weather, particularly when the site is
disturbed. Although most cases of histoplasmosis are
mild or even unnoticed, this disease can, in rare cases,
cause blindness and/or death. Individuals who are
weakened by other health conditions or who do not have
endemic immunity are at greater risk from histoplasmosis.
Starlings also compete
with native cavity-nesting birds such as bluebirds,
flickers, and other woodpeckers, purple martins, and
wood ducks for nest sites. One report showed that, where
nest cavities were limited, starlings had severe impacts
on local populations of native cavity-nesting species.
One author has speculated that competition with
starlings may cause shifts in red-bellied woodpecker (Melanerpes
carolinus) nesting from urban habitats to rural forested
areas where starling competition is less.
Legal Status
European starlings are not
protected by federal law and in most cases not by state
law. Laws vary among states, however, so check with
state wildlife officials before beginning a control
program. In addition, state or local laws may regulate
or prohibit certain control techniques such as shooting
or the use of toxicants.
Damage Prevention and Control Methods
Exclusion
Close all
openings larger than 1 inch (2.5 cm) to exclude
starlings from buildings or other structures. This is a
permanent solution to problems inside the structure
(Fig. 4). Heavy plastic (polyvinyl chloride, PVC) or
rubber strips hung in open doorways of farm buildings
have been successful in some areas in excluding birds
while allowing people, machinery, or livestock to enter.
Hang 10-inch (25-cm) wide strips with about 2.0-inch
(5-cm) gaps between them. These strips might also be
useful for protecting feed bunks. Netting over doorways
may also exclude birds from buildings, but would be
easily torn by machinery or livestock.
Where starlings are
roosting or nesting on the ledge of a building, place a
wooden, metal, or plexiglass covering over the ledge at
a 45o angle to prevent use (Fig. 5). Metal protectors or
porcupine wires (Nixalite® and Cat Claw®) are also
available for preventing roosting on ledges or roof
beams.
Nylon or plastic netting
is another option for exclusion (Fig. 6). Exclude
starlings that are roosting inside open farm buildings
by covering the underside of the roof beams with
netting. Netting is also useful for covering fruit crops
such as cherries or grapes to prevent bird damage, and
studies show it to be a cost-effective method of
protecting higher-value grapes in commercial vineyards.
For wine grapes harvested one time per season,
tractor-mounted rollers can facilitate installation and
removal of netting draped directly over vines. Some New
York vineyards have used this method for five years with
the original netting still in good condition. For table
grapes harvested by hand several times per year, a frame
can be used to hold the netting above the vines so it
doesn’t interfere with the frequent harvests. A
practical tip: if protecting the total vineyard is
impractical, protect varieties that receive the most
damage, those that ripen early or are otherwise highly
attractive to birds (for example, small, dark, sweet
grapes.)
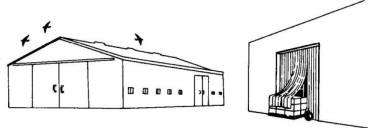
Fig. 4. Bird-proof buildings to permanently eliminate
bird problems inside.
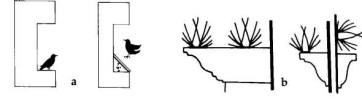
Fig. 5. A wooden, metal, or plexiglass covering over a
ledge at a 45° angle (a) or porcupine wires (b) can be
used to prevent roosting and nesting.
 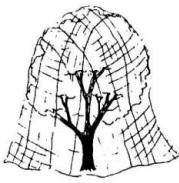
Fig. 6. Netting can be used to exclude birds from
building rafters and from fruit trees.
Where starlings compete
with other birds for nest boxes, proper nest box
construction helps. For bluebird boxes, use a round 1
1/2-inch (3.8-cm) hole or a rectangular slot, 4 inches
(10 cm) wide by 1 1/8 inches (29 mm) high, to allow
bluebirds in but keep starlings out. Starlings are
discouraged by horizontal wood duck nest boxes made from
a 24-inch (61-cm) section of 12-inch-diameter (30.5-cm)
stove pipe. The ends are made from wooden circles, and
the entrance hole on one end is semicircular and 4
inches (10 cm) high by 11 inches (28 cm) wide. Other
nest box features such as interior dimensions and color,
amount of light allowed into the box, and box placement
appear to have potential for discouraging starlings
while encouraging preferred cavity-nesters.
Cultural Methods and Habitat Modification
Livestock Facilities.
Starlings are attracted to livestock operations by the
food and water that is available to them. Feedlots offer
an especially attractive food source to starlings during
winter when snow cover and frozen ground impede their
normal feeding in open fields or other areas. The snow
cover and frozen ground increase the likelihood as well
as the severity of damage.
Some livestock operations
are more attractive to starlings than others. Operations
that have large quantities of feed always available,
especially when located near a starling roost, are the
most likely to have damage problems. Research results
emphasize the importance of farm management practices in
long-term starling control. These practices limit the
availability of food and water to starlings, thus making
the livestock environment less attractive to birds. The
following practices used singly, but preferably in
combination, will reduce feed losses, the chance of
disease transmission, and the cost and labor of
conventional control measures.
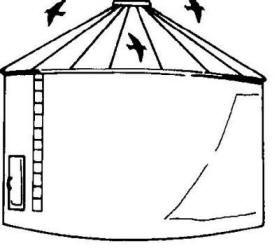 Fig.
7. Use bird-proof facilities to store grain. Fig.
7. Use bird-proof facilities to store grain.
1. Clean up spilled grain.
2. Store grain in bird-proof facilities (Fig. 7).
3. Use bird-proof livestock feeders. These include
flip-top pig feeders, lick wheels for liquid cattle
supplement, and automatic-release feeders (magnetic or
electronic) for costly high-protein rations. Using
covered feeders prevents starling access and
contamination of the food source, and the banging of the
lift-top lids as pigs use the feeders may frighten
starlings and keep them uneasy. Avoid feeding on the
ground because this is an open invitation to starlings.
-
Where possible, feed
livestock in covered areas such as open sheds
because these areas are less attractive to
starlings.
-
Use feed forms that
starlings cannot swallow, such as cubes or blocks
greater than 1/2 inch (1.3 cm) in diameter. Minimize
use of 3/16 inch (0.5 cm) pellets; starlings consume
these six times faster than granular meal.
-
When feeding protein
supplements with other rations, such as silage, mix
them well to limit starling access to the
supplements.
-
Where possible, adjust
feeding schedules so that exposure of feed to birds
is minimized. For example, when feeding once per
day, such as in a limited energy-feeding program for
gestating sows, delay the feeding until late in the
afternoon when foraging by starlings is decreased.
Feed cattle at night if possible.
-
Starlings prefer to
feed early to midday and in areas where feed is
constantly available. Feeding schedules that take
these factors into account minimize problems.
Starlings are especially attracted to water. Drain
or fill in unnecessary water pools around livestock
operations. Where feasible, control the water level
of livestock waterers to make them unavailable or
less attractive to starlings (Fig. 8).
Tree Roosts. When
roosts occur in a small number of landscape trees near
homes or along streets, thinning branches from the trees
used by birds will usually disperse them. Roosts in tree
groves or woodlots usually occur in dense, overcrowded
stands of young trees. Remove about one-third of the
trees to improve the tree stand, especially if marked by
a professional forester, and to disperse the birds. Such
thinning successfully dispersed roosts from research
woodlots in Ohio and Kentucky, and from at least two
problem-roost situations in Nebraska. In dense cedar
thickets, bulldozing strips through the roost to remove
one-third of the habitat has also been successful in
dispersing birds. Soil disturbance, however, may be
hazardous if soils harbor fungal spores of the human
respiratory disease histoplasmosis. For further
information on roost dispersal, see Bird Dispersal
Techniques.
Frightening
Frightening is
effective in dispersing starlings from roosts,
small-scale fruit crops, and some other troublesome
sites. It is useful around livestock operations that
have warm climates year-round, and where major
concentrations of wintering starlings exist. In the
central states, starlings concentrate at livestock
facilities primarily during cold winter months when snow
covers natural food sources. At this time, baiting and
other techniques are generally more effective than
frightening. In addition, frightening starlings may
disperse birds to other livestock facilities, a negative
point that should be considered if disease transfer is a
concern.
Frightening devices
include recorded distress or alarm calls, gas-operated
exploders, battery-operated alarms, pyrotechnics (shellcrackers,
bird bombs), chemical frightening agents (see Avitrol®
below), lights (for roosting sites at night), bright
objects, and various other stimuli. Some novel visual
frightening devices with potential effectiveness are
eye-spot balloons, hawk kites, and mylar reflective
tape. Ultrasonic (high frequency, above 20 kHz) sounds
are not effective in frightening starlings and most
other birds because, like humans, they do not hear these
sounds.
Harassing birds throughout
the evening as they land can be effective in dispersing
bird roosts if done for three to four consecutive
evenings or until birds no longer return. Spraying birds
with water from a hose or from sprinklers mounted in the
roost trees has helped in some situations. Beating on
tin sheets or barrels with clubs also scares birds. A
combination of several scare techniques used together
works better than a single technique used alone. Vary
the location, intensity, and types of scare devices to
increase their effectiveness. Two additional tips for
successful frightening efforts: 1) begin early before
birds form a strong attachment to the site, and 2) be
persistent until the problem is solved. For a more
detailed discussion of frightening techniques, see Bird
Dispersal Techniques.
Avitrol®. Avitrol® (active
ingredient: 4-aminopyridine) is a Restricted Use
Pesticide available in several bait formulations for use
as a chemical frightening agent. It is for sale only to
certified applicators or persons under their direct
supervision and only for those uses covered by the
applicator’s certification.
Avitrol® baits contain a
small number of treated grains or pellets mixed with
many untreated grains or pellets. Birds that eat the
treated portion of the bait behave erratically and/or
give warning cries that frighten other birds from the
area. Generally, birds that eat the treated particles
will die. Avitrol® baits are available for controlling
starlings at feedlots and structures. At the dilution
rates registered for use at feedlots, there is a low but
potential hazard to nontarget hawks and owls that might
eat birds killed by Avitrol®. It is therefore important
to pick up and bury or incinerate any dead starlings
found.
Around livestock
operations, Avitrol® is sometimes used where the goal is
to frighten or disperse the birds rather than to kill
them. However, many birds may be killed, and data are
lacking on whether the results of Avitrol® use at
feedlots occur because of frightening aspects or from
direct mortality.
Three Avitrol®
formulations are labeled for starling control at
feedlots (Pelletized Feed, Double Strength Corn Chops,
and Powder Mix). The formulation most appropriate for a
given situation may vary, particularly if large numbers
of blackbirds are mixed with the starlings. However, the
Pelletized Feed formulation is generally recommended for
starling control because starlings usually prefer
pellets over cracked corn (corn chops). The Double
Strength Corn Chops formulation is probably best for
mixed flocks of starlings and blackbirds. Because
Avitrol® is designed as a frightening agent, birds can
develop bait shyness (bait rejection) fairly quickly.
Prebaiting for several days with untreated pellets may
be necessary for effective bait consumption and control.
If starling problems persist, changing bait locations
and additional prebaiting may be needed. If any Avitrol®
baits are to be used, contact a qualified person trained
in bird control work (someone from USDA-APHIS-ADC or
Cooperative Extension, for example) for technical
assistance.
Repellents
Soft, sticky
repellents such as Roost-No-More®, Bird Tanglefoot®,
4-The-Birds®, and others consist of polybutenes, a
nontoxic material that can be useful in discouraging
starlings from roosting on sites such as ledges, roof
beams, or shopping-center signs. It is often helpful to
first put masking tape on the surface needing
protection, then apply the repellent onto the tape; this
increases effectiveness on porous surfaces and makes
removal easier. Over time, these materials lose their
effectiveness and have to be replaced.
Recent research has found
that flavoring used in grape soft drinks, dimethyl
anthranilate (DMA), and methyl anthranilate (MA) repel
starlings from livestock feed at rates that do not
affect cattle. Although subsequent field trials showed
that DMA may not be cost-effective in some situations,
results have indicated that MA has potential for
cost-effective starling repellency.
Research is ongoing to
improve the cost-effectiveness of this compound and to
develop its potential for managing starlings at
livestock facilities and possibly for repelling birds
from fruit crops.
Toxicants
When using
toxicants or other pesticides, always refer to the
current pesticide label and follow its instructions as
the final authority on pesticide use.
Starlicide. A chemical
compound developed for starling control during the 1960s
by the Denver Wildlife Research Center is now
commercially available as a pelletized bait. It is sold
under the trade name Starlicide Complete (0.1% 3-chloro
p-toluidine hydrochloride).
Starlicide is a
slow-acting toxicant for controlling starlings and
blackbirds around livestock and poultry operations. It
is toxic to other types of birds in differing amounts,
but will not kill house (English) sparrows (Passer
domesticus) at registered levels. Mammals are generally
resistant to its toxic effects.
Poisoned birds experience
a slow, nonviolent death. They usually die from 1 to 3
days after feeding, often at their roost. Generally, few
dead starlings will be found at the bait site. Poisoned
starlings are not dangerous to scavengers or predators.
However, to provide good sanitation and to prevent the
spread of diseases that the birds may carry, pick up and
bury or incinerate any dead starlings.
It is important to use
fresh bait, as the current formulation of Starlicide
Complete loses effectiveness in storage. Bait kept on
hand from one winter to the next may lose some of its
potency, and bait kept for 2 years may not work at all.
How to Use. Field
tests in both the western and eastern United States have
established guidelines for using Starlicide. For the
best success in a control program, we recommend the
following steps:
1. Observe birds feeding
in and around the livestock operation. Note the number
of starlings and when and where they prefer to feed. The
best time for observing is usually during the first few
hours following sunrise when birds are seeking their
morning meal.
-
Determine what species
of birds are feeding. If any protected birds, such
as doves, quail, pheasants, or songbirds, are
present, do not apply toxic bait. For assistance or
advice on bird identification or nontarget risk
assessment based on the situation, contact your
local Cooperative Extension office, USDA-APHIS-ADC
office, or the state wildlife agency.
-
Prebait, for best
results, with a nonpoisonous bait to accustom
starlings to feeding on bait at particular
locations. Place the prebait in areas where the
starlings concentrate to feed, but where it will not
be accessible to livestock or other nontarget
animals. The best prebait is a high-quality food
that resembles the toxic bait in color, size, and
texture. If such prebait is unavailable, use a good
quality feed such as that normally fed to livestock.
Prebait for 1 to 4 days
until the birds readily feed on the prebait. If good
consumption is not obtained, move the prebait to another
location where starlings are concentrating to feed.
-
Apply prebait and bait
on cold days when snow covers the ground. This
timing is more effective because starlings become
stressed for food and concentrate in livestock
feeding areas.
-
Place prebait and bait
in containers to ensure proper bait placement and to
protect it from the weather (Fig. 9). Black rubber
calf feeder pans work well. They do not tip easily,
their dark color does not frighten birds, and bait
is openly exposed. Empty farm wagons, feeder lids
turned upside down, wooden troughs, or other
containers may also work. Avoid brightly colored or
shiny containers or ones that might tip and spill
bait. At night, the containers can be covered to
protect the bait from the weather. However, they
must be uncovered at dawn so that starlings can feed
as soon as they arrive. At feedlots where large
numbers of starlings (more than 100,000) are
involved, and where large quantities of feed are
available on the ground, broadcasting bait in
alleyways as per label directions is recommended.
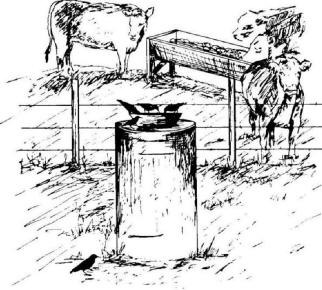
Fig. 9. Well-positioned
bait containers, excluded from livestock, provide better
safety and control in baiting programs for starlings.
-
Apply toxic bait after
starlings feed readily on the prebait by removing
all prebait and replacing it with the toxic bait.
Consult the label directions for the amount to use
(1 pound [0.45 kg] of Starlicide Complete used
properly will kill about 100 to 200 starlings). The
total number of starlings using a farm over a long
period of time may greatly exceed the numbers
observed on a given day, so continue baiting for at
least 2 or 3 days or until bait consumption
diminishes. Bait should be available to the
starlings at all times when they are present.
-
Good bait acceptance
may be more difficult to obtain in warm-weather
climates such as in the southernmost states. If this
occurs, and the Starlicide Complete bait is not
eaten, an alternative may be to use Starlicide
Technical (98% active ingredient) applied to baits
such as french-fried potatoes, small fruits, or
livestock feed according to label directions. The
french fries and fruits may be more attractive to
starlings, but they can spoil rapidly. Generally,
livestock feed makes an acceptable bait because
starlings are accustomed to feeding on it.
Starlicide Technical can be used only by or under
supervision of the USDA-APHIS-ADC for control of
blackbirds and starlings at livestock operations.
Contact them for help.
-
Remove bait after bait
consumption diminishes. Observe any birds arriving
at the feedlot during the next 2 to 3 mornings after
baiting. Reduced bird numbers at this time indicate
control, as most birds will die at the roost. If
starlings continue to be present, or if they
gradually return in increasing numbers, wait until a
number of birds are regularly returning to feed at
the area. Then apply prebait and toxic bait (Steps 4
to 6) as before. Do not leave Starlicide baits
exposed for prolonged periods because this may cause
bait shyness (bait rejection), and may also increase
hazards to protected bird species.
-
Group baiting may
increase effectiveness. Consider coordinating
control efforts with your neighbors. Several persons
baiting at the same time will produce better control
because starlings may forage over a large
geographical area and may change feeding sites from
day to day. Notify local wildlife officials of your
plans so that if large numbers of starlings are
removed, the officials will be able to explain the
die-off. Contact USDA-APHIS-ADC officials about the
possibility of using roost control procedures if a
large roost is associated with the damage problem.
9. Cautions: Starlicide is
poisonous to chickens, turkeys, ducks, and some other
birds. Never expose bait where poultry, livestock, or
nontarget wildlife can feed on it. Do not repackage
pesticides into anything other than their original
containers. Read and follow all label directions.
Toxic Perches.
Toxic perches are perforated metal tubes 24 or 27 inches
(61 or 69 cm) long containing a wick saturated with a
contact toxicant that enters the feet as the birds perch
on the tube. They can be safely and effectively used in
certain industrial and other structural roost situations
where they do not present hazards to nontarget birds and
avian predators such as hawks and owls. All killed birds
should be picked up immediately and buried or burned
because of potential hazards to other wildlife.
The active ingredient in
toxic perches, fenthion (Rid-A-Bird Perch 1100
Solution), is federally registered as a Restricted Use
Pesticide for use in these perches. Fenthion is rapidly
absorbed through the skin and should be used with
caution to avoid spillage and exposure to the handler.
It is toxic to humans, birds, fish, and aquatic
invertebrates. For additional information on fenthion,
refer to Pesticides.
Agents for Roost
Control. Roost control has been used to reduce
starling damage at livestock feedlots and in urban areas
where there are human health and sanitation concerns. A
recent study indicates, however, that roost control with
wetting agents (no longer registered) may not
consistently provide long-term reduction of birds at
feedlots, despite mostly favorable results in reducing
urban problems. The presence of other roosting
populations near the treated roost may be an important
factor. At urban sites having mild winter climates, the
accumulation of bird carcasses can produce a severe odor
and fly problem if carcasses are not picked up or buried
at the site soon after roost treatment. Bulldozing sites
is the most efficient method to bury carcasses, but soil
disturbance during this process may present human health
hazards from dissemination of histoplasmosis spores.
Such roost control should be considered only as a last
resort when other alternatives are not likely to solve
the problem in livestock and urban roost situations.
Currently, the only
material registered for roost control is Starlicide
Technical, which is used for baiting at or near roost
sites in areas where starlings congregate before
roosting. This method is currently registered for use in
only a few states and only under supervision of
USDA-APHIS-ADC personnel. A federal registration is
pending. Although this method of roost control is
labor-intensive, it has been effective.
Fumigants
Fumigation is
generally not practical for starling control, and no
fumigants are registered for this purpose.
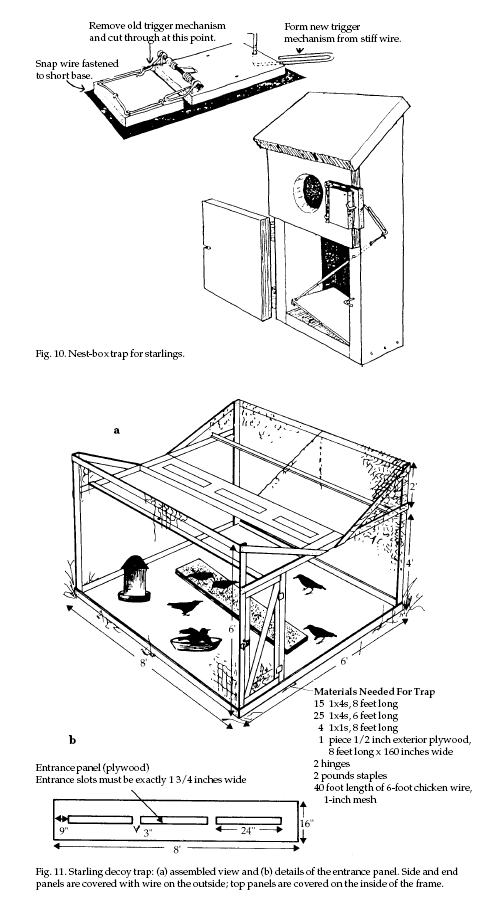 Trapping Trapping
Trapping and
removing starlings can be a successful method of control
at locations where a resident population is causing
localized damage or where other techniques cannot be
used. An example is trapping starlings in a fruit
orchard.
Two types of traps,
nest-box and decoy traps, are commonly used. Nest-box
traps (Fig. 10) are successful only during the nesting
season, whereas decoy traps (Fig. 11) are most effective
during other times when the birds are flocking.
Nontarget birds captured in traps should be immediately
released unharmed.
Decoy traps for starlings
should be at least 5 to 6 feet (1.5 to 1.8 m) high to
allow for servicing and can be quite large (for example,
10 feet [3 m] wide by 30 feet [9 m] long). A convenient
size is 6 x 8 x 6 feet (1.8 x 2.4 x 1.8 m) (Fig. 11). If
desired, the sides and top can be constructed in panels
to facilitate transportation and storage. In addition,
decoy traps can be set up on a farm wagon and thereby
moved to the best places to catch starlings. Place traps
where starlings are likely to congregate. Leave a few
starlings in the trap as decoys; their feeding behavior
and calls attract other starlings that are nearby. Decoy
birds in the trap must be well watered (which may
include a bird bath) and fed. A well-maintained decoy
trap can capture 100 or more starlings per day depending
on its size and location, the time of year, and how well
the trap is maintained. Euthanize captured starlings
humanely such as by carbon dioxide exposure or cervical
dislocation.
Shooting
Shooting is
more effective as a dispersal technique than as a way to
reduce starling numbers. The number of starlings that
can be killed by shooting is very small in relation to
the number of starlings usually involved in pest
situations. Shooting, however, can be helpful to
supplement and reinforce other dispersal techniques. For
more detail on dispersal, see Bird Dispersal Techniques.
Economics of Damage and Control
Consumption of livestock
feed by starlings can at times be a substantial economic
consideration. Data reported in 1968 from Colorado
feedlots estimated the cost of cattle rations consumed
during winter by starlings at $84 per 1,000 starlings.
Current feed costs and the associated losses would
certainly be much higher. A 1967 report indicated that 1
million starlings at a California feedlot resulted in
losses of $1,000 per day because of food consumption and
contamination, and starling interference with cattle
feeding Fig. 11. Starling decoy trap: (a) assembled view
and (b) details of the entrance panel. Side and end
activity. Another report estimated panels are covered
with wire on the outside; top panels are covered on the
inside of the frame.
that starlings in Idaho
consumed 15 to 20 tons (13.5 to 18 mt) of cattle feed
per day. A 1978 study in England estimated that the food
eaten by starlings in a calf-rearing unit over three
winters was 6% to 12% of the food presented to the
calves. Two other studies in England since then found 4%
losses and negligible damage, respectively.
Producers who wish to
estimate feed losses to starlings at their facilities
can do so using one of two methods. The following
equation, which was developed from data in Colorado,
estimates the cost of feed consumed per day:
Cost of feed ration
consumed per day = estimated starlings (to the nearest
1,000) x fraction of birds using trough x cost of feed
ration per pound (0.4536 kg) x 0.0625 pound (0.02813 kg)
consumed per starling per day.
A second method, which may
be applicable to most geographic areas, precludes the
need of estimating starling populations. It requires the
operator to observe the feed troughs several times
during the day and estimate the number of starlings
entering the troughs per day. From this estimate the
cost of the feed ration consumed per day can be
estimated with the following equation:
Cost of feed ration
consumed per day = estimated starling entries into
troughs x 0.0033 pounds (0.0015 kg) consumed per
starling entry x cost of feed ration per pound (0.4536
kg). These losses projected over a 3-to 4-month damage
season can assist in evaluating the costs and benefits
of proposed control measures.
Feed contamination from
starling excreta may not be an economic loss for cattle
or pig operations. In 2 years of testing at Western
Kentucky University, neither pigs nor cattle were
adversely affected by long-term exposure to feed heavily
contaminated with starling excreta. As compared to
controls, no significant differences were observed in
weight gain or feed efficiency (ratio of weight gain to
weight of feed offered). In addition, there were no
observed differences in feed rejection or disease
incidence. These results indicate that there is no
economic justification for starling control based solely
on feed contamination. However, the effects of livestock
water contamination from starling excreta have not been
well studied.
Starling interference with
livestock feeding patterns may have economic importance.
A study in England reported that calves in pens
protected from starlings showed higher growth rates and
better feed conversion than those in unprotected pens.
This protection led to an increased profit margin. The
difference observed, however, might have been caused by
starlings in the unprotrected pens consuming the calf
food, especially the high protein portion, rather than
by actual interference with the calf feeding.
The costs associated with
starlings in the spread of livestock disease may at
times be substantial. For example, during the severe
winter of 1978-1979, a TGE outbreak occurred in
southeast Nebraska, with over 10,000 pigs lost in 1
month in Gage County alone. Starlings were implicated
because the TGE outbreak was concurrent with large
flocks of starlings feeding at the same facilities. More
recent data show that starlings are capable of carrying
this disease in their feces. The role of starlings in
disease transfer, however, needs further study.
Bird damage to grapes in
the United States was estimated to be at least $4.4
million in 1972; starlings were one of the species
causing the most damage. Starlings, as well as many
other species of birds, also damage ripening cherry
crops. A 1972 study in Michigan found 17.4% of a total
crop lost to birds. A 1975 study in England estimated
damage at 14% (lower branches) to 21% (tree canopy) of
the crop; similar 1976 data showed less damage. Starling
damage to winter wheat in a study of 218 fields in three
regions in Kentucky and Tennessee averaged 3.8%, 0.5%,
and 0.4% respectively, with the most serious losses
(more than 14%) occurring where wheat was planted late
and fields were within 11 miles (16 km) of a large
starling roost.
Human health and safety
problems associated with urban starling roosts include
concerns about the disease histoplasmosis and about
aircraft-bird collisions. Although serious problems
occur only infrequently, they can have grievous
consequences where loss of human life and/or permanent
disability may occur. Moreover, equipment repair and
replacement costs associated with aircraft-bird
collisions can be substantial. For example, the costs of
aircraft-bird collisions in the United States are
estimated to be at least $20 million per year to
commercial aircraft and $10 million per year to Air
Force aircraft. These consequences mandate a thorough
understanding of urban roost situations and timely roost
management where the potential for human health and
safety problems exists.
On the beneficial side,
starlings eat large quantities of insects and other
invertebrates, especially during spring. Many of these
invertebrates, such as lawn grubs, are considered to be
pests. This benefit, however, is partially offset by the
fact that starlings often take over nest cavities of
native insect-eating birds. As trends move toward lower
pesticide use and sustainable, low-input turf and
agricultural systems, the role of starlings and other
birds may become more important. Research is needed to
further understand potential positive impacts of
starlings and to learn how to maximize potential
benefits while minimizing problems.
Although starlings are
frequently associated with damage problems, some of
which clearly cause substantial economic losses, the
economics of damage in relation to the cost and
effectiveness of controls are not well understood.
Several factors contribute to this: (1) Starlings are
difficult to monitor because they often move long
distances daily from roost to feeding areas, and many
migrate. (2) Effectiveness of controls, particularly in
relation to the total population in an area, is
difficult to document. For example, does population
reduction in a particular situation reduce the problem
or merely allow an influx of starlings from other areas,
and how does this vary seasonally or annually? In
addition, does lethal control just substitute for
natural mortality or is it additive?
(3) The economics of
interactions with other species are difficult to
measure. For example, how much is a bluebird or flicker
worth, and what net benefits occur when starling
interference with native cavity-nesting birds is
considered? (4) Other factors such as weather and
variation among problem situations complicates accurate
evaluation of damage and the overall or long-term
effectiveness of controls. These points, as well as
others mentioned in this chapter, are examples of
factors that must be considered in assessing the total
economic impact of starlings. Clearly, to minimize
starling-human conflicts we need a better understanding
of starlings and their interactions with various
habitats and control measures.
Acknowledgments
The references listed
under “For Additional Information” and many others were
used in preparing this chapter. Gratitude is extended to
the authors and the many researchers and observers who
contributed to this body of knowledge. We also thank M.
Beck, J. Besser, R. Fritschen, D. Mott, A. Stickley, and
R. Timm for comments on the first edition of this
chapter, J. Andelt provided typing and technical
assistance. We gratefully acknowledge M. Beck, R. Case,
D. Mott, and A. Stickley for critical reviews of this
second edition, and L. Germer, J. Gosey, and D. Reese
for reviews of specific portions.
Figure 1 from US Fish and
Wildlife Service (1974), “Controlling Starlings,”
Bulletin AC 209, Purdue University, West Lafayette,
Indiana, modified and adapted by Renee Lanik, University
of Nebraska-Lincoln.
Figure 2 from Bystrak et
al. (1974), used with permission. Figure copyrighted by
the National Audubon Society, Inc. Adapted by David
Thornhill. Map by J. W. Rosahn, based on the National
Audubon Society’s annual Christmas Bird Count. Map
reprinted by permission from “Wintering Areas of Bird
Species Potentially Hazardous to Aircraft.” D. Bystrak
et al. (1974), National Audubon Society, Inc.
Figure 3 photo by Ron J.
Johnson.
Figures 4 and 7 by Renee
Lanik based on drawings by Jon Eggers and a drawing from
Salmon and Gorenzel’s chapter “Cliff Swallows” in this
publication.
Figure 5 by Renee Lanik,
University of Nebraska-Lincoln.
Figure 6 by Jill Sack
Johnson.
Figures 8 and 9 by Renee
Lanik, University of Nebraska-Lincoln.
Figure 10 from DeHaven and
Guarino (1969), adapted by Jill Sack Johnson.
Figure 11 by Renee Lanik
based on E. R. Kalmbach (1939), “The Crow in Its
Relation to Agriculture,” US Dep. Agric. Farmer’s Bull.
No. 1102, rev. ed., Washington, DC. 21 pp., and US Fish
Wildl. Serv. (no date), “Trapping Starlings,” Bull. AC
210, Purdue Univ., West Lafayette, Indiana.
For Additional Information
Aubin, T. 1989. The role
of frequency modulation in the process of distress calls
recognition by the starling (Sturnus vulgaris). Behav.
108:57-72.
Barnes, T. G. 1991.
Eastern bluebirds, nesting structure design and
placement. College of Agric. Ext. Publ. FOR-52, Univ.
Kentucky, Lexington. 4 pp.
Besser, J. F., J. W.
DeGrazio, and J. L. Guarino. 1968. Costs of wintering
starlings and red-winged blackbirds at feedlots. J.
Wildl. Manage. 32:179-180.
Besser, J. F., W. C.
Royall, Jr., and J. W. DeGrazio. 1967. Baiting starlings
with DRC-1339 at a cattle feedlot. J. Wildl. Manage.
31:45-51.
Blokpoel, H. 1976. Bird
hazards to aircraft. Clarke, Irwin & Co. Ltd., 236 pp.
Boudreau, C. W. 1975. How
to win the war with pest birds. Wildl. Tech., Hollister,
California. 174 pp.
Bystrak, D., C. S.
Robbins, S. R. Drennan, and R. Arbib, eds. 1974.
Wintering areas of bird species potentially hazardous to
aircraft. A special report jointly prepared by the Natl.
Audubon Soc. and the US Fish Wildl. Serv. Natl. Audubon
Soc., Inc., New York. 156 pp.
Constantin, B., and J.
Glahn. 1989. Controlling roosting starlings in
industrial facilities by baiting. Proc. Eastern Wildl.
Damage Control Conf. 4:47-52.
Crase, F. T., C. P. Stone,
R. W. DeHaven, and D. F. Mott. 1976. Bird damage to
grapes in the United States with emphasis on California.
US Fish Wildl. Serv., Special Sci. Rep. - Wildl. No.
197. Washington, DC. 18 pp.
DeCino, T. J., D. J.
Cunningham, and E. W. Schafer, Jr. 1966. Toxicity of
DRC-1339 to starlings. J. Wildl. Manage. 30:249-253.
DeHaven, R. W., and J. L.
Guarino. 1969. A nest-box trap for starlings.
Bird-Banding 40:49-50.
Dolbeer, R. A. 1984.
Blackbirds and starlings: population ecology and habits
related to airport environments. Proc. Wildl. Hazards to
Aircraft Conf. Training Workshop. Charleston, South
Carolina, US Dep. Trans. Rep. DOT/FAA/AAS/84-1:149-159.
Dolbeer, R. A. 1989.
Current status and potential of lethal means of reducing
bird damage in agriculture. Pages 474-483 in H. Ouellet,
ed., Acta XIX Congressus Int. Ornithol., Vol. I. Univ.
Ottawa Press, Ottawa, Canada.
Dolbeer, R. A., A. R.
Stickley, Jr., and P. P. Woronecki. 1978/1979. Starling,
Sturnus vulgaris, damage to sprouting wheat in Tennessee
and Kentucky, U.S.A. Prot. Ecol. 1:159-169.
Dolbeer, R. A., P. P.
Woronecki, R. L. Bruggers. 1986. Reflecting tapes repel
blackbirds from millet, sunflowers, and sweet corn.
Wildl. Soc. Bull. 14:418-425.
Dolbeer, R. A., P. P.
Woronecki, A. R. Stickley, Jr., and S. B. White. 1978.
Agricultural impacts of a winter population of
blackbirds and starlings. The Wilson Bull. 90:31.
Feare, C. J. 1980. The
economics of starling damage. Pages 39-55 in E. N.
Wright, I. R. Inglis, and C. J. Feare, eds. Bird
problems in agriculture, British Crop Prot. Council,
Croydon, UK. 210 pp.
Feare, C. J. 1984. The
starling. Oxford Univ. Press, New York. 315 pp.
Feare, C. J. 1989. The
changing fortunes of an agricultural bird pest: the
European starling. Agric. Zool. Rev. 3:317-342.
Feare, C. J., and K. P.
Swannack. 1978. Starling damage and its prevention at an
open-fronted calf yard. An. Prod. 26:259-265.
Fuller-Perrine, L. D., and
M. E. Tobin. 1993. A method for applying and removing
bird-exclusion netting in commercial vineyards. Wildl.
Soc. Bull. 21:47-51.
Glahn, J. F. 1982. Use of
starlicide to reduce starling damage at livestock
feeding operations. Proc. Great Plains Wildl. Damage
Control Workshop 5:273-277.
Glahn, J. F., and D. L.
Otis. 1986. Factors influencing blackbird and European
starling damage at livestock feeding operations. J.
Wildl. Manage. 50:15-19.
Glahn, J. F., and W.
Stone. 1984. Effects of starling excrement in the food
of cattle and pigs. An. Prod. 38:439-446.
Glahn, J. F., S. K.
Timbrook, and D. J. Twedt. 1987. Temporal use patterns
of wintering starlings at a southeastern livestock farm:
implications for damage control. Proc. Eastern Wildl.
Damage Control Conf. 3:194-203.
Glahn, J. F., D. J. Twedt,
and D. L. Otis. 1983. Estimating feed loss from starling
use of livestock feed troughs. Wildl. Soc. Bull.
ll:366-372.
Glahn, J. F., A. R.
Stickley, Jr., J. F. Heisterberg, and D. F. Mott. 1991.
Impact of roost control on local urban and agricultural
blackbird problems. Wildl. Soc. Bull. 19:511-522.
Good, H. B., and D. M.
Johnson. 1978. Nonlethal blackbird roost control. Pest
Control 46:14ff.
Gough, P. M., and J. W.
Beyer. 1982. Bird-vectored diseases. Proc. Great Plains
Wildl. Damage Control Workshop 5:260-272.
Heisterberg, J. F., A. R.
Stickley, Jr., K. M. Garner, and P. D. Foster, Jr. 1987.
Controlling blackbirds and starlings at winter roosts
using PA-14. Proc. Eastern Wildl. Damage Control Conf.
3:177-183.
Holler, N. R., and E. W.
Schafer, Jr. 1982. Potential secondary hazards of
avitrol baits to sharp-shinned hawks and American
kestrels. J. Wildl. Manage. 46:457-462.
Ingold, D. J. 1989.
Nesting phenology and competition for nest sites among
red-headed and red-bellied woodpeckers and European
starlings. Auk 106:209-217.
Johnson, R. J., P. K.
Cole, and W. W. Stroup. 1985. Starling response to three
auditory stimuli. J. Wildl. Manage. 49:620-625.
Johnson, R. J., and J. F.
Glahn. 1992. Starling management in agriculture. Coop.
Ext. Publ. NCR 451, Univ. Nebraska, Lincoln. 7pp.
Lefebvre, P. W., and J. L.
Seubert. 1970. Surfactants as blackbird stressing
agents. Proc. Vertebr. Pest Conf. 4:156-161.
Lumsden, H. G. 1986.
Choice of nest boxes by tree swallows, Tachycineta
bicolor, house wrens, Troglodytes aedon, eastern
bluebirds, Sialia sialis, and European starlings,
Sturnus vulgaris. Can. Field-Nat. 100:343-349.
Lustick, S. 1976. Wetting
as a means of bird control. Proc. Bird Control Sem.
7:41-47.
Maccarone, A. D. 1987.
Effect of snow cover on starling activity and foraging
patterns. Wilson Bull. 99:94-97.
Mason, J. R., J. F. Glahn,
R. A. Dolbeer, and R. F. Reidinger. 1985. Field
evaluation of dimethyl anthranilate as a bird repellent
livestock feed additive. J. Wildl. Manage. 49:636-642.
Meanley, B., and W. C.
Royall, Jr. 1976. Nationwide estimates of blackbirds and
starlings. Proc. Bird Control Sem. 7:39-40.
McGilvrey, F. B., and F.
M. Uhler. 1971. A starling-deterrent wood duck nest box.
J. Wildl. Manage. 35:793-797.
Palmer, T. K. 1976. Pest
bird damage in cattle feedlots: the integrated systems
approach. Proc. Vertebr. Pest Conf. 7:17-21.
Royall, W. C., Jr., T. C.
DeCino, and J. F. Besser. 1967. Reduction of a starling
population at a turkey farm. Poultry Sci. 46:1494-1495.
Schmidt, R. H., and R. J.
Johnson. 1984. Bird dispersal recordings: an overview.
Am. Soc. Testing Mat. STP 817, Philadelphia,
Pennsylvania 4:43-65 .
Shirota, Y., M. Sanada,
and S. Masaki. 1983. Eyespoted balloons as a device to
scare gray starlings. Appl. Ent. Zool. 18:545-549.
Stables, E. R., and N. D.
New. 1968. Birds and aircraft: the problems. Pages 3-16
in R. K. Murton and E. N. Wright, eds. Problems of birds
as pests. Acad. Press, London.
Stewart, P. A. 1978.
Foraging behavior and length of dispersal flights of
communally roosting starlings. Bird-Banding 49:113-115.
Stickley, A. R., Jr. 1979.
Extended use of starlicide in reducing bird damage in
southeastern feedlots. Proc. Bird Control Sem. 8:79-81.
Stickley, A. R., Jr., D.
J. Twedt, J. F. Heisterberg, D. F. Mott, and J. F. Glahn.
1986. Surfactant spray system for controlling blackbirds
and starlings in urban roosts. Wildl. Soc. Bull.
14:412-418.
Stickley, A. R., Jr., and
R. J. Weeks. 1985. Histoplasmosis and its impact on
blackbird-starling roost management. Proc. Eastern Wildl.
Damage Control Conf. 2:163-171.
Twedt, D. J., and J. F.
Glahn. 1982. Reducing starling depredations at livestock
feed operations through changes in management practices.
Proc. Vertebr. Pest Conf. 10:159-163.
Weitzel, N. H. 1988.
Nest-site competition between the European starling and
native breeding birds in northwestern Nevada. Condor
90:515-517.
West, R. R. 1968.
Reduction of a winter starling population by baiting its
preroosting areas. J. Wildl. Manage. 32:637-640.
West, R. R., J. F. Besser,
and J. W. DeGrazio. 1967. Starling control in livestock
feeding areas. Proc. Vertebr. Pest Conf., 3:89-93.
Will, T. J. 1983. BASH
Team new developments. Proc. Bird Control Sem. 9:7-10.
Wright, E. N. 1973.
Experiments to control starling damage at intensive
animal husbandry units. European Plant Prot. Organiz.
Bull. 9:85-89.
Wright, E. N. 1982. Bird
problems and their solutions in Britain. Proc. Vertebr.
Pest Conf. 10:186-189.
Editors
Scott E. Hygnstrom Robert
M. Timm Gary E. Larson
PREVENTION AND CONTROL OF
WILDLIFE DAMAGE — 1994
Cooperative Extension
Division Institute of Agriculture and Natural Resources
University of Nebraska -Lincoln
United States Department
of Agriculture Animal and Plant Health Inspection
Service Animal Damage Control E-109
Great Plains Agricultural
Council Wildlife Committee
01/08/2007
Special
thanks to:
Clemson University
|